Why are buffers important to living things
Why Are Buffers Important To Living Things. In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms. The movement of h+ ions in acidic or alkaline solutions can damage the cells. Since many chemical reactions and enzymatic activity in living organisms require specific ph for optimum function, buffers are essential to keep organisms functioning. Doesn’t matter if the solution is in a beaker or your blood, that is what they do.
 Why are buffers important? From proquestyamaha.web.fc2.com
Why are buffers important? From proquestyamaha.web.fc2.com
A buffer is a mixture a molecules that acts to keep the ph or a solution close to neutral. Apart from the stomach and small intestine, the normal ph of body sollutions is 7.4, and it is critical to survival that it doesn’t stray too far from tha. The movement of h+ ions in acidic or alkaline solutions can damage the cells. In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms. They allow the cells to maintain ph stability when put in alkaline or acidic solutions. So buffers help to keep the ph of solutions stable.
Buffer is a chemical substance that maintains the ph of a biological system when a small amount.
This is because buffers maintain the right ph of a liquid. Buffers are extremely important to living organisms because all biochemical processes proceed normally only when the ph remains close to 7. Why are buffers important to living things quizlet? Buffers keep the ph of the solution stable when acids or bases are added. In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms. Buffers are essential for living cells.
 Source: proquestyamaha.web.fc2.com
Source: proquestyamaha.web.fc2.com
Phosphate buffer maintains the internal environment of cells. Why are buffers important to living things? So buffers help to keep the ph of solutions stable. Buffers are extremely important to living organisms because all bioch… ckbear14 ckbear14 09/07/2015 english high school answered why are buffers important to living things? Small molecules such as bicarbonate and phosphate provide buffering.
 Source: sciencing.com
Source: sciencing.com
This is because buffers maintain the right ph of a liquid. Phosphate buffer maintains the internal environment of cells. The movement of h+ ions in acidic or alkaline solutions can damage the cells. Small molecules such as bicarbonate and phosphate provide buffering. Buffers keep the ph of the solution stable when acids or bases are added.
 Source: roundtaiwanround.com
Source: roundtaiwanround.com
The movement of h+ ions in acidic or alkaline solutions can damage the cells. Buffers are chemical solutions that resist the change in ph( hydrogen ion concentration in a solution). Buffers are extremely important to living organisms because all bioch… ckbear14 ckbear14 09/07/2015 english high school answered why are buffers important to living things? This is because buffers maintain the right ph of a liquid. Why are buffers important to living things?
 Source: slideserve.com
Source: slideserve.com
Small molecules such as bicarbonate and phosphate provide buffering. So buffers help to keep the ph of solutions stable. Buffers are essential for living cells. This is because buffers maintain the right ph of a liquid. Buffers \textbf{buffers} buffers are important to living things for maintaining blood ph and because many biochemical reactions like metabolism, respiration, muscle relaxation, nerve impulse transmission take place only within a narrow ph range.
 Source: acidsandbases-101.weebly.com
Source: acidsandbases-101.weebly.com
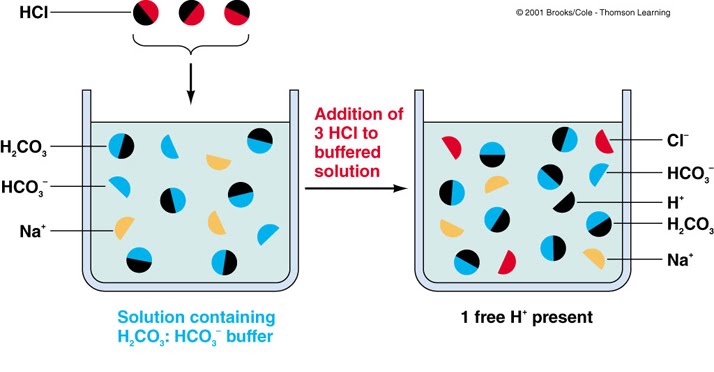
So buffers help to keep the ph of solutions stable. This is because buffers maintain the right ph of a liquid. What is a buffer and why are they important to living things? Buffers \textbf{buffers} buffers are important to living things for maintaining blood ph and because many biochemical reactions like metabolism, respiration, muscle relaxation, nerve impulse transmission take place only within a narrow ph range. When a small quantity of an acid or base is added, the buffer behaves in such a way, to.
 Source: davey.com
Source: davey.com
Why are buffers important to living things? How do buffers protect living things? In humans, for example, buffers act to maintain blood ph between 7.35 and 7.45 even though acids and bases are continually being added to and removed from the blood as it travels through the body. Buffers are extremely important to living organisms because all biochemical processes proceed normally only when the ph remains close to 7. Doesn’t matter if the solution is in a beaker or your blood, that is what they do.
 Source: slideserve.com
Source: slideserve.com
Buffers keep the ph of the solution stable when acids or bases are added. In humans, for example, buffers act to maintain blood ph between 7.35 and 7.45 even though acids and bases are continually being added to and removed from the blood as it travels through the body. A buffer is a mixture a molecules that acts to keep the ph or a solution close to neutral. Buffers keep the ph of the solution stable when acids or bases are added. This is because buffers maintain the right ph of a liquid.
 Source: es.slideshare.net
Source: es.slideshare.net
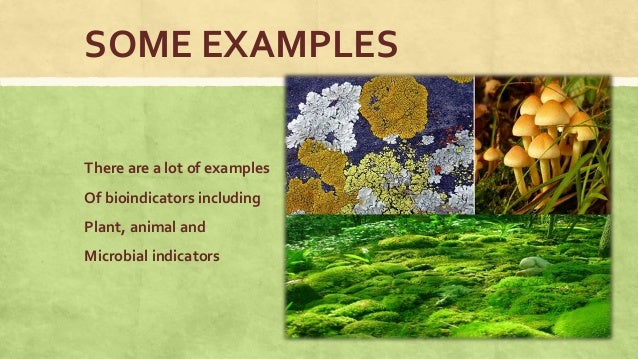
This is because buffers maintain the right ph of a liquid. Buffers are extremely important to living organisms because all biochemical processes proceed normally only when the ph remains close to 7. Apart from the stomach and small intestine, the normal ph of body sollutions is 7.4, and it is critical to survival that it doesn’t stray too far from tha. Small molecules such as bicarbonate and phosphate provide buffering. Why are buffers important to living things?

In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms. Phosphate buffer maintains the internal environment of cells. How do buffers protect living things? When a small quantity of an acid or base is added, the buffer behaves in such a way, to. Since many chemical reactions and enzymatic activity in living organisms require specific ph for optimum function, buffers are essential to keep organisms functioning.
 Source: slideshare.net
Source: slideshare.net
Buffer is a chemical substance that maintains the ph of a biological system when a small amount. Buffers are chemical solutions that resist the change in ph( hydrogen ion concentration in a solution). Phosphate buffer maintains the internal environment of cells. A buffer is a mixture of molecules that acts to keep the ph of a solution close to neutral. Buffers are extremely important to living organisms because all bioch… ckbear14 ckbear14 09/07/2015 english high school answered why are buffers important to living things?
 Source: slideserve.com
Source: slideserve.com
Buffers are extremely important to living organisms because all biochemical processes proceed normally only when the ph remains close to 7. Small molecules such as bicarbonate and phosphate provide buffering. Why are buffers important to living things quizlet? Since many chemical reactions and enzymatic activity in living organisms require specific ph for optimum function, buffers are essential to keep organisms functioning. Buffers are essential for living cells.
 Source: ehow.com
Source: ehow.com
Buffers are extremely important to living organisms because all bioch… ckbear14 ckbear14 09/07/2015 english high school answered why are buffers important to living things? So buffers help to keep the ph of solutions stable. Buffer is a chemical substance that maintains the ph of a biological system when a small amount. Doesn’t matter if the solution is in a beaker or your blood, that is what they do. When a small quantity of an acid or base is added, the buffer behaves in such a way, to.
 Source: ehow.com
Source: ehow.com
In humans, for example, buffers act to maintain blood ph between 7.35 and 7.45 even though acids and bases are continually being added to and removed from the blood as it travels through the body. Why are buffers important to living things quizlet? A buffer is a mixture of molecules that acts to keep the ph of a solution close to neutral. Buffers are chemical solutions that resist the change in ph( hydrogen ion concentration in a solution). They allow the cells to maintain ph stability when put in alkaline or acidic solutions.
 Source: es.slideshare.net
Source: es.slideshare.net
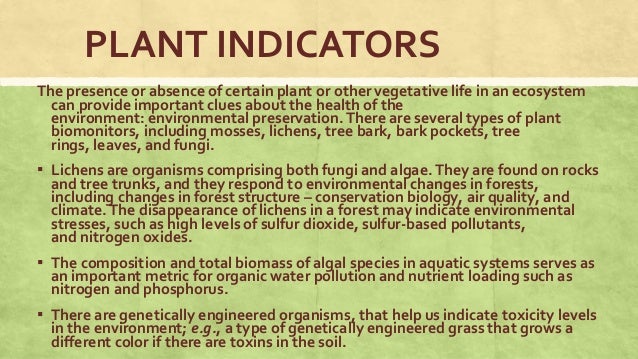
Why are buffers important to living things? This is because buffers maintain the right ph of a liquid. In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms. Why are buffers important to living things? Buffers keep the ph of the solution stable when acids or bases are added.
 Source: reference.com
Source: reference.com
A buffer is a mixture of molecules that acts to keep the ph of a solution close to neutral. Buffers are chemical solutions that resist the change in ph( hydrogen ion concentration in a solution). This is because buffers maintain the right ph of a liquid. Buffers \textbf{buffers} buffers are important to living things for maintaining blood ph and because many biochemical reactions like metabolism, respiration, muscle relaxation, nerve impulse transmission take place only within a narrow ph range. In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms.
 Source: proquestyamaha.web.fc2.com
Source: proquestyamaha.web.fc2.com
How do buffers protect living things? Phosphate buffer maintains the internal environment of cells. Why are buffers important to living things? In humans, for example, buffers act to maintain blood ph between 7.35 and 7.45 even though acids and bases are continually being added to and removed from the blood as it travels through the body. Since many chemical reactions and enzymatic activity in living organisms require specific ph for optimum function, buffers are essential to keep organisms functioning.
 Source: psiberg.com
Source: psiberg.com
Doesn’t matter if the solution is in a beaker or your blood, that is what they do. Doesn’t matter if the solution is in a beaker or your blood, that is what they do. Additionally dramatic shifts in ph can play a role in controlling cellular activities such. A buffer is a mixture of molecules that acts to keep the ph of a solution close to neutral. In living organisms buffers are important because they resist sudden changes in the ph of body fluids of living organisms.
 Source: sciencing.com
Source: sciencing.com
So buffers help to keep the ph of solutions stable. The movement of h+ ions in acidic or alkaline solutions can damage the cells. Buffers are extremely important to living organisms because all biochemical processes proceed normally only when the ph remains close to 7. They allow the cells to maintain ph stability when put in alkaline or acidic solutions. Small molecules such as bicarbonate and phosphate provide buffering.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why are buffers important to living things by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.