Which element has the highest ionization energy
Which Element Has The Highest Ionization Energy. The tabular chart on the right is arranged by ionization energy. * argon is a novel gas. Therefore, boron (b) has the highest i.e. For chemistry students and teachers:

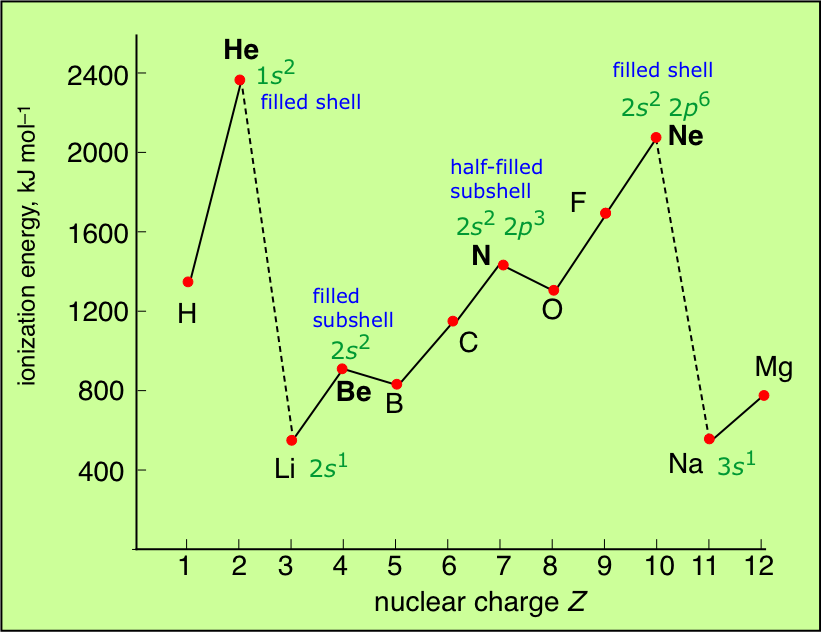
The ionization energy decreases from top to bottom within a group. If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements. Which element from the following has the highest ionization energy? Which of the following has the highest ionization energy? Elements and its ionization energies are given. * ionisation energy increases as we move from left to right in a period.
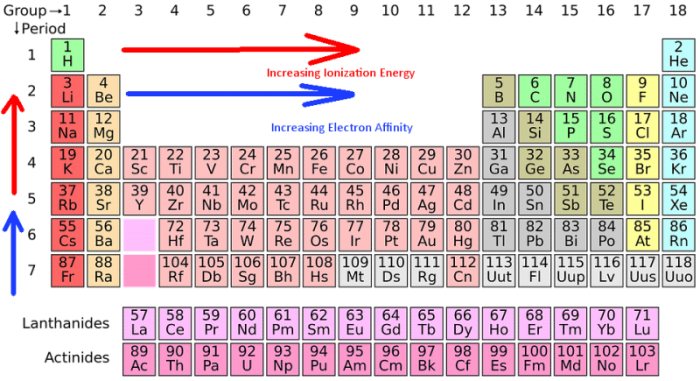
* ionisation energy increases as we move from left to right in a period.
Therefore, the ionization energy increases from left to as the number of. Third ionisation enthalpy means you have to remove third electon from1s, as it has duplet configuration and small ionic size in dipositive state. * ionisation energy increases as we move from left to right in a period. Argon presents in the right most column. Therefore, the ionization energy increases from left to as the number of. Periodic properties of the elements.

Li has the smallest value of n, so it has the highest ionization energy 4 consider the given options as the number of protons in the nucleus increases, the ionization energy increases as well; Na (11) or cs (55)? Na (11) or cs (55)? Thus fluorine has a stronger nuclear force on the surrounding electrons and requires more energy to remove electrons. The unity for ionization energy is ev.
 Source: youtube.com
Source: youtube.com
In the 3rd period argon(ar) has the highest ionisation energy. Third ionisation enthalpy means you have to remove third electon from1s, as it has duplet configuration and small ionic size in dipositive state. From this trend, caesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization. Electronegativity, ionization energy, atomic radius, and electron affinity. The unity for ionization energy is ev.
 Source: brainly.com
Source: brainly.com
Thus fluorine has a stronger nuclear force on the surrounding electrons and requires more energy to remove electrons. Be has electronic configuration 1s2 2s2. It is very difficult to remove an electron from a fluorine atom. The first chemical element is cesium and the last one is helium. From this trend, caesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization.
 Source: pinterest.com
Source: pinterest.com
Answer which element has the highest ionization energy? It is very difficult to remove an electron from a fluorine atom. Be has electronic configuration 1s2 2s2. For chemistry students and teachers: That too be has small atomic size among alkaline earth metals

Fluorine has the highest ionization energy among halogen family as it is at a lower energy level and does not receive the same intensity of shielding as iodine. For chemistry students and teachers: The first ionization energy of magnesium is larger than sodium because magnesium has one more proton in its nucleus to hold on to the electrons in the 3s orbital. To list the elements order by ionization energy, click on the table headers. Fluorine has the highest ionization energy among halogen family as it is at a lower energy level and does not receive the same intensity of shielding as iodine.
 Source: therealgroupichem.weebly.com
Source: therealgroupichem.weebly.com
In the fifth group, ionization potential (i.e) decreases on moving down the group from top to bottom. Third ionisation enthalpy means you have to remove third electon from1s, as it has duplet configuration and small ionic size in dipositive state. 120 rows ionization energy chart of all the elements is given below. Elements and its ionization energies are given. Periodic properties of the elements.
 Source: socratic.org
Source: socratic.org
Li has the smallest value of n, so it has the highest ionization energy 4 consider the given options as the number of protons in the nucleus increases, the ionization energy increases as well; The unity for ionization energy is ev. You can print the list of elements by hitting the print button below. Na k li cs these elements are all in the same column of the periodic table; In the fifth group, ionization potential (i.e) decreases on moving down the group from top to bottom.
 Source: sciencetrends.com
Source: sciencetrends.com
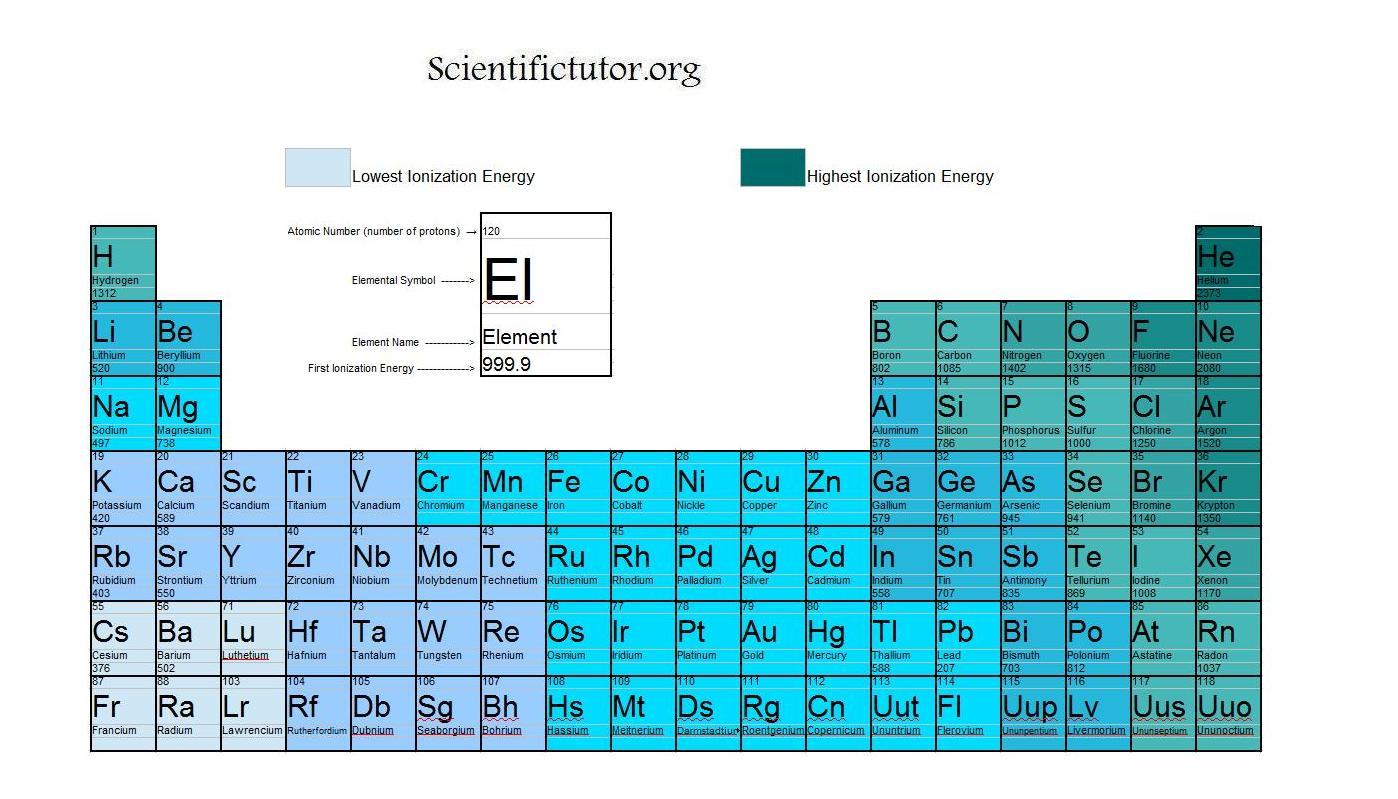
This is due to two reasons: 120 rows ionization energy chart of all the elements is given below. Electronegativity, ionization energy, atomic radius, and electron affinity. To list the elements order by ionization energy, click on the table headers. It is very easy to remove an electron from a francium atom.

Periodic properties of the elements. Answer which element has the highest ionization energy? Which element from the following has the highest ionization energy? From this trend, caesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization. Periodic properties of the elements.
 Source: gy.kimiq.com
Source: gy.kimiq.com
The first ionization energy of magnesium is larger than sodium because magnesium has one more proton in its nucleus to hold on to the electrons in the 3s orbital. * argon is a novel gas. Third ionisation enthalpy means you have to remove third electon from1s, as it has duplet configuration and small ionic size in dipositive state. * ionisation energy increases as we move from left to right in a period. The ionization energy decreases from top to bottom within a group.
 Source: kemichemist2.blogspot.com
Source: kemichemist2.blogspot.com
Therefore, the ionization energy increases from left to as the number of. From this trend, caesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization. Na (11) or cs (55)? Therefore, boron (b) has the highest i.e. Fluorine has the highest ionization energy among halogen family as it is at a lower energy level and does not receive the same intensity of shielding as iodine.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Fluorine has the highest ionization energy among halogen family as it is at a lower energy level and does not receive the same intensity of shielding as iodine. In the fifth group, ionization potential (i.e) decreases on moving down the group from top to bottom. The unity for ionization energy is ev. To list the elements order by ionization energy, click on the table headers. Be has electronic configuration 1s2 2s2.
 Source: nemoquiz.com
Source: nemoquiz.com
It is very easy to remove an electron from a francium atom. You can print the list of elements by hitting the print button below. * argon is a novel gas. Therefore, boron (b) has the highest i.e. Fluorine has the highest ionization energy among halogen family as it is at a lower energy level and does not receive the same intensity of shielding as iodine.
 Source: brainly.com
Source: brainly.com
- ionisation energy increases as we move from left to right in a period. You can print the list of elements by hitting the print button below. Elements and its ionization energies are given. The unity for ionization energy is ev. Answer which element has the highest ionization energy?
 Source: sukachem.blogspot.com
Source: sukachem.blogspot.com
The ionization energy decreases from top to bottom within a group. Helium (he) has the highest ionization energy, then neon (ne) ionization energy increases as you go across a period from left to right. Answer which element has the highest ionization energy? Na (11) or cs (55)? Be has electronic configuration 1s2 2s2.

Periodic properties of the elements. In the 3rd period argon(ar) has the highest ionisation energy. That too be has small atomic size among alkaline earth metals From this trend, caesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization. Therefore, boron (b) has the highest i.e.

The ionization energy decreases from top to bottom within a group. If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements. In the fifth group, ionization potential (i.e) decreases on moving down the group from top to bottom. Li has the smallest value of n, so it has the highest ionization energy 4 consider the given options as the number of protons in the nucleus increases, the ionization energy increases as well; Therefore, boron (b) has the highest i.e.

Electronegativity, ionization energy, atomic radius, and electron affinity. 120 rows ionization energy chart of all the elements is given below. It is very difficult to remove an electron from a fluorine atom. Helium (he) has the highest ionization energy, then neon (ne) ionization energy increases as you go across a period from left to right. * ionisation energy increases as we move from left to right in a period.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which element has the highest ionization energy by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.