Magnesium bicarbonate chemical formula
Magnesium Bicarbonate Chemical Formula. The chemical equation for the reaction can be given as,. Mgco 3 + 2 hcl →. Magnesium bicarbonate contains magnesium, hydrogen, carbon and oxygen. The chemical formula for magnesium bicarbonate is mg(hco3)2 what are the elements present in magnesium bicarbonate?
 Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses From chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses From chemistrylearner.com
The chemical formula for magnesium bicarbonate is mg(hco3)2 what are the elements present in magnesium bicarbonate? Or if any of the following reactant substances co2 (carbon dioxide), disappearing Mg(oh) 2 + 2 co 2 → mg(hco 3) 2 mg(hco 3) 2 → mgco 3 + co 2 + h 2 o chemical properties with acids. Like many common group 2 metal carbonates, magnesium carbonate reacts with aqueous acids to release carbon dioxide and water: So magnesium bicarbonate must have two bicarbonate ions. Mgoh 2 2co 2 mghco 3 2 h 2 o mgco 3 co 2 mghco 3 2 view all equations with mghco32 as product.
Mgco 3 + 2 hcl →.
Indeed, let’s learn about chemical formula and uses of magnesium carbonate here: At high temperature mgco3 will decompose to magnesium oxide and carbon dioxide by reaction as below: Mgco3 mgo + co2 (h = +118 kj / mol) you can also read: Magnesium bicarbonate contains magnesium, hydrogen, carbon and oxygen. Mgco3 is an inorganic salt in the form of white crystals. Mg(oh) 2 + 2 co 2 → mg(hco 3) 2 mg(hco 3) 2 → mgco 3 + co 2 + h 2 o chemical properties with acids.
 Source: pinterest.com
Source: pinterest.com
Mgco3 is an inorganic salt in the form of white crystals. At high temperature mgco3 will decompose to magnesium oxide and carbon dioxide by reaction as below: Magnesite consists of white trigonal crystals. Write the formula of the following compound: When magnesium chloride is reacted with sodium bicarbonate, magnesium carbonate, sodium chloride, water and carbon dioxide are formed.
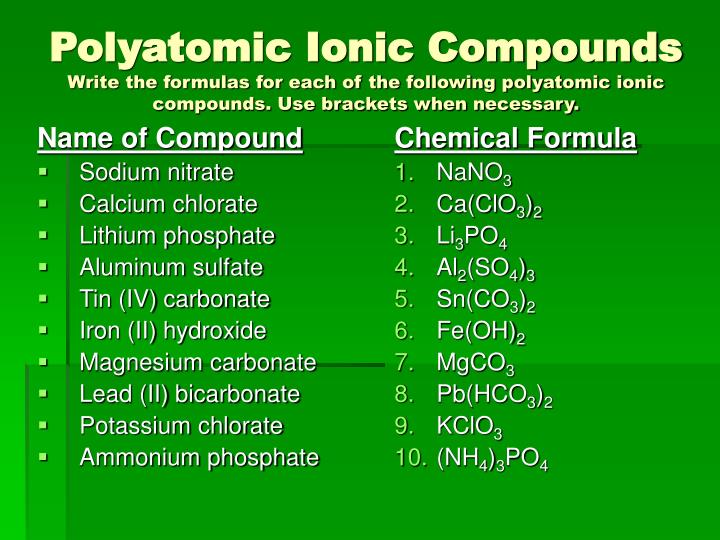 Source: slideserve.com
Source: slideserve.com
So magnesium bicarbonate must have two bicarbonate ions. ️ mg(hco3)2 | magnesium bicarbonate = h2o | water + mgco3 | magnesium carbonate + co2 | carbon dioxide | nhiệt độ nhiệt độ, nhiệt phân muối mg(hco3)2 chuyển thành muối mgco3 không tan., có hiện tượng mgco3 kết tủa trắng, đồng thời có khí co2 thoát ra. Write the formula of the following compound: Or if any of the following reactant substances co2 (carbon dioxide), disappearing 100% (3 ratings) transcribed image text:
 Source: slideshare.net
Source: slideshare.net
Magnesium bicarbonate contains magnesium, hydrogen, carbon and oxygen. The magnesium carbonate formula is mgco 3. The chemical formula of magnesium ore, dolomite can be represented as mgco 3 ·caco 3. Or if any of the following reactant substances co2 (carbon dioxide), disappearing Mgco3 mgo + co2 (h = +118 kj / mol) you can also read:
 Source: google.com
Source: google.com
The magnesium carbonate formula is mgco 3. Mgco3 mgo + co2 (h = +118 kj / mol) you can also read: At high temperature mgco3 will decompose to magnesium oxide and carbon dioxide by reaction as below: Type the part of name or the formula of substance for search: When magnesium chloride is reacted with sodium bicarbonate, magnesium carbonate, sodium chloride, water and carbon dioxide are formed.
 Source: brainly.in
Source: brainly.in
️ mg(hco3)2 | magnesium bicarbonate = h2o | water + mgco3 | magnesium carbonate + co2 | carbon dioxide | nhiệt độ nhiệt độ, nhiệt phân muối mg(hco3)2 chuyển thành muối mgco3 không tan., có hiện tượng mgco3 kết tủa trắng, đồng thời có khí co2 thoát ra. This equation does not have any specific information about phenomenon. This reference contains the names of substances and descriptions of the chemical formulas (including the structural formula and the skeletal formula). ️ mg(hco3)2 | magnesium bicarbonate = h2o | water + mgco3 | magnesium carbonate + co2 | carbon dioxide | nhiệt độ nhiệt độ, nhiệt phân muối mg(hco3)2 chuyển thành muối mgco3 không tan., có hiện tượng mgco3 kết tủa trắng, đồng thời có khí co2 thoát ra. The chemical formula for magnesium bicarbonate is mg(hco3)2 what are the elements present in magnesium bicarbonate?
 Source: brainly.in
Source: brainly.in
The chemical formula for magnesium bicarbonate is mg(hco3)2 what are the elements present in magnesium bicarbonate? It is slightly more alkaline than acidic. Reactions in which magnesium bicarbonate is involved. This equation does not have any specific information about phenomenon. Magnesium bicarbonate contains magnesium, hydrogen, carbon and oxygen.
 Source: slideserve.com
Source: slideserve.com
Mgoh 2 2co 2 mghco 3 2 h 2 o mgco 3 co 2 mghco 3 2 view all equations with mghco32 as product. It is an alkaline earth metal carbonate. Write the formula of the following compound: The bicarbonate is then vacuum dried, causing it to lose carbon dioxide and a molecule of water: This equation does not have any specific information about phenomenon.
 Source: brainly.in
Source: brainly.in
It is an alkaline earth metal carbonate. The most common magnesium carbonate form is the anhydrous salt known as the magnesite. So magnesium bicarbonate must have two bicarbonate ions. The magnesium carbonate formula is mgco 3. Rotaract international of lexington, ky.
 Source: lidolearning.com
Source: lidolearning.com
It is slightly more alkaline than acidic. At high temperature mgco3 will decompose to magnesium oxide and carbon dioxide by reaction as below: The chemical formula of magnesium ore, dolomite can be represented as mgco 3 ·caco 3. Like many common group 2 metal carbonates, magnesium carbonate reacts with aqueous acids to release carbon dioxide and water: It is an alkaline earth metal carbonate.
 Source: google.com
Source: google.com
Rotaract international of lexington, ky. At high temperature mgco3 will decompose to magnesium oxide and carbon dioxide by reaction as below: When magnesium chloride is reacted with sodium bicarbonate, magnesium carbonate, sodium chloride, water and carbon dioxide are formed. It is an alkaline earth metal carbonate. ️ mg(hco3)2 | magnesium bicarbonate = h2o | water + mgco3 | magnesium carbonate + co2 | carbon dioxide | nhiệt độ nhiệt độ, nhiệt phân muối mg(hco3)2 chuyển thành muối mgco3 không tan., có hiện tượng mgco3 kết tủa trắng, đồng thời có khí co2 thoát ra.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Mgco3 is an inorganic salt in the form of white crystals. It is an alkaline earth metal carbonate. So magnesium bicarbonate must have two bicarbonate ions. Magnesium bicarbonate contains magnesium, hydrogen, carbon and oxygen. Write the formula of the following compound:
 Source: biocoherence.eu
Source: biocoherence.eu
Mg(oh) 2 + 2 co 2 → mg(hco 3) 2 mg(hco 3) 2 → mgco 3 + co 2 + h 2 o chemical properties with acids. Mgco 3 + 2 hcl →. The chemical equation for the reaction can be given as,. Or if any of the following reactant substances co2 (carbon dioxide), disappearing In this case, you just need to observe to see if product substance mg(hco3)2 (magnesium bicarbonate), appearing at the end of the reaction.
 Source: people.uwec.edu
Source: people.uwec.edu
Magnesium bicarbonate contains magnesium, hydrogen, carbon and oxygen. Mgoh 2 2co 2 mghco 3 2 h 2 o mgco 3 co 2 mghco 3 2 view all equations with mghco32 as product. As shown in the chemical formula magnesium carbonate is conjugated with calcium carbonate. The chemical formula of magnesium ore, dolomite can be represented as mgco 3 ·caco 3. Like many common group 2 metal carbonates, magnesium carbonate reacts with aqueous acids to release carbon dioxide and water:
 Source: pristinerevival.com
Source: pristinerevival.com
The magnesium carbonate formula is mgco 3. 100% (3 ratings) transcribed image text: Mg(oh) 2 + 2 co 2 → mg(hco 3) 2 mg(hco 3) 2 → mgco 3 + co 2 + h 2 o chemical properties with acids. The chemical formula for magnesium bicarbonate is mg(hco3)2 what are the elements present in magnesium bicarbonate? The chemical formula of magnesium carbonate is ({\rm{mgc}}{{\rm{o}}_3}).
 Source: google.com
Source: google.com
The most common magnesium carbonate form is the anhydrous salt known as the magnesite. The chemical formula of magnesium carbonate is ({\rm{mgc}}{{\rm{o}}_3}). Mgcl 2(aq) + 2nahco 3(aq) →mgco 3(s) + 2nacl (aq) + h. When magnesium chloride is reacted with sodium bicarbonate, magnesium carbonate, sodium chloride, water and carbon dioxide are formed. This equation does not have any specific information about phenomenon.
 Source: google.com
Source: google.com
Mgco 3 + 2 hcl →. Or if any of the following reactant substances co2 (carbon dioxide), disappearing So magnesium bicarbonate must have two bicarbonate ions. Type the part of name or the formula of substance for search: Mgco3 is an inorganic salt in the form of white crystals.
 Source: byjus.com
Source: byjus.com
The magnesium carbonate formula is mgco 3. In this case, you just need to observe to see if product substance mg(hco3)2 (magnesium bicarbonate), appearing at the end of the reaction. Mgco 3 + 2 hcl →. The chemical formula for magnesium bicarbonate is mg(hco3)2 what are the elements present in magnesium bicarbonate? At high temperature mgco3 will decompose to magnesium oxide and carbon dioxide by reaction as below:
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title magnesium bicarbonate chemical formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






