How many moles are in 22 grams of argon
How Many Moles Are In 22 Grams Of Argon. Mole is the amount of the substance that contains the same number of particles. Therefore, number of moles =. Thus, we can conclude that there are 0.55 moles in 22 grams of argon. The number of moles is the substance of an element divided by its molar mass.
 Argon How Many Moles Are In 22 Grams Of Argon From argonnekonba.blogspot.com
Argon How Many Moles Are In 22 Grams Of Argon From argonnekonba.blogspot.com
Based on the formula above, we created the moles of argon to grams converter below. The number of moles is the substance of an element divided by its molar mass. 607g / 40g moles or 15.175 moles. You can view more details on each measurement unit: What is the number of moles in 22 grams of argon? Here is the moles of argon to grams formula:
Molecular weight of argon or mol the molecular formula for argon is ar.
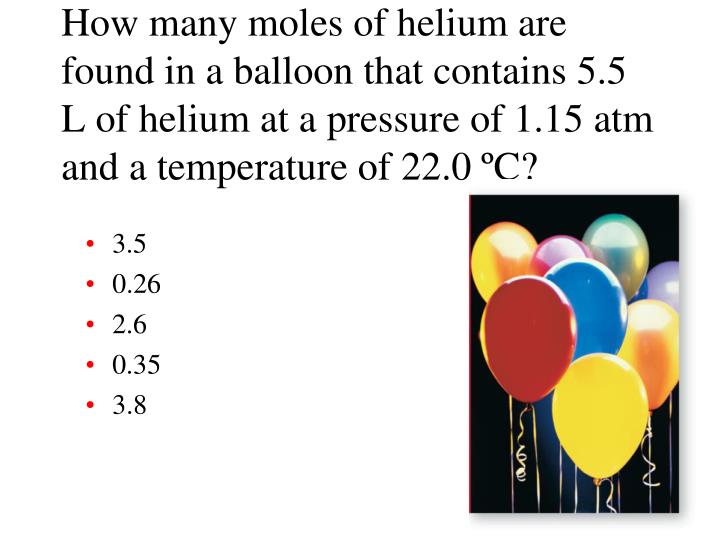
What is the number of moles in 22 grams of argon? The si base unit for amount of substance is the mole. For example, if argon has a mass of 22 grams, and mgo has a mass of 88.1 grams, then the atoms in argon have a molar masses of 6.022×1023 (molar mass of a molecule). Mathematically, number of moles =. It is known that number of moles equal mass divided by molar mass. How many grams are in a mole of argon?
 Source: slideshare.net
Source: slideshare.net
22 grams of argon is equivalent to 0,55 moles. Mathematically, number of moles =. 5 moles of argon contain 30,110704285.10e23 molecules. We assume you are converting between grams argon and mole. 607g / 40g moles or 15.175 moles.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
It means that one mole of argon weighs 40g. How many grams is 5.00 atoms of iron? How many molecules are in 5 moles of argon? The number of moles is the substance of an element divided by its molar mass. 1 grams argon is equal to 0.025032542304996 mole.
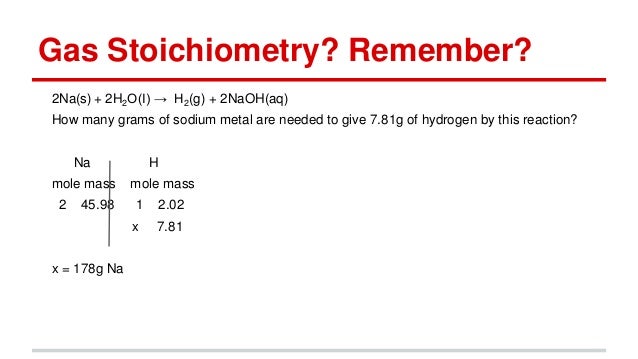
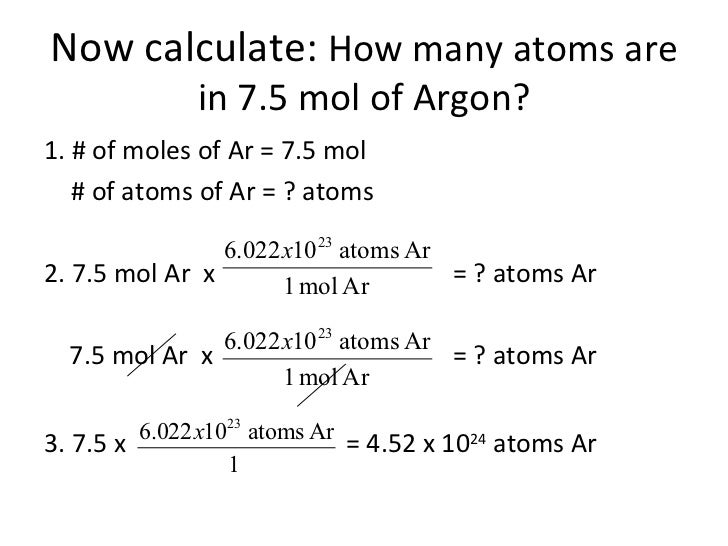 Source: slideshare.net
Source: slideshare.net
So, if we have a light source with a molar mass of 2.00*10’m, then we can remove the electrons in. Therefore, number of moles =. How.many moles are in 22 grams of argon? Can you please help me with number 7. 22 grams of argon is equivalent to 0,55 moles.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
We assume you are converting between grams argon and mole. Mole is the amount of the substance that contains the same number of particles. We assume you are converting between grams argon and mole. For example, if argon has a mass of 22 grams, and mgo has a mass of 88.1 grams, then the atoms in argon have a molar masses of 6.022×1023 (molar mass of a molecule). Molecular weight of argon or mol the molecular formula for argon is ar.
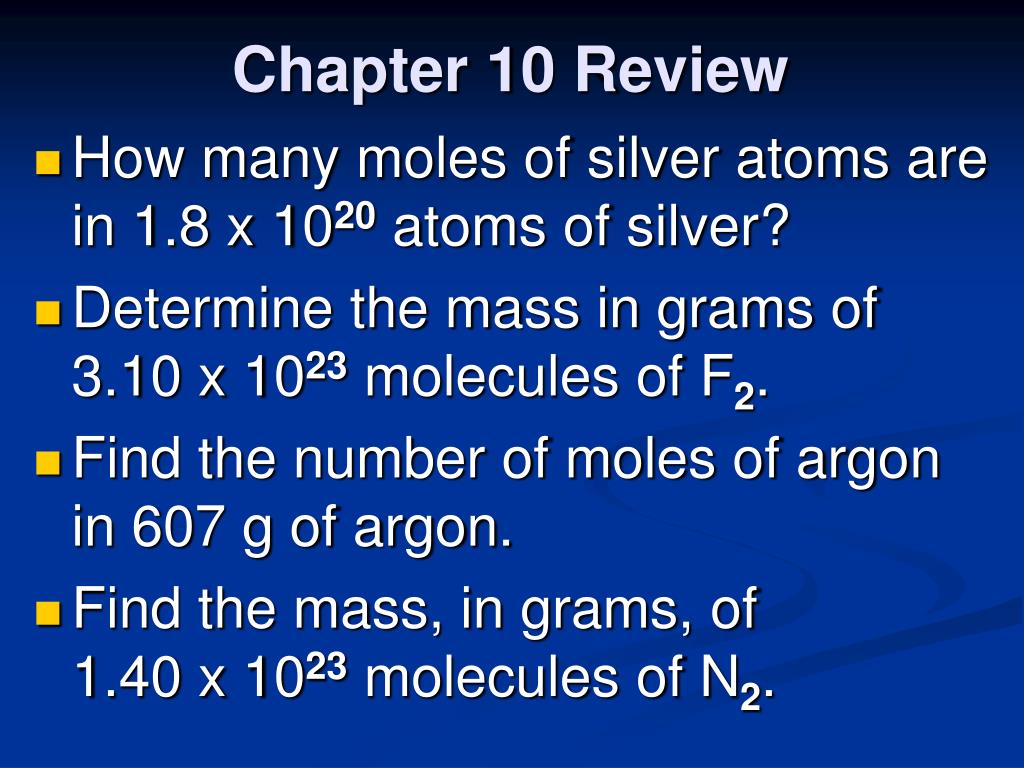 Source: slideserve.com
Source: slideserve.com
770 grams 3 how many moles are in 22 grams of argon. The number of moles is the substance of an element divided by its molar mass. You can view more details on each measurement unit: 22 grams of argon is equivalent to 0,55 moles. 1 grams argon is equal to 0.025032542304996 mole.
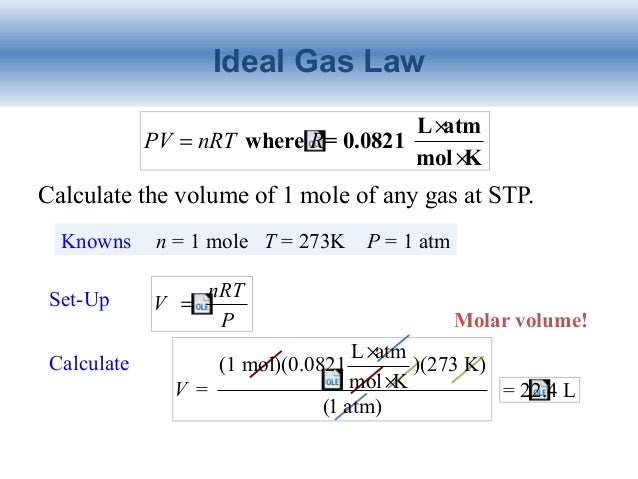 Source: nitrogengassanroso.blogspot.com
Source: nitrogengassanroso.blogspot.com
To solve this let us find out the molar mass of argon, it is 39.948 g/mol or roughly 40 g /mol. 607g / 40g moles or 15.175 moles. What is the number of moles in 22 grams of argon? 22 grams of argon is equivalent to 0,55 moles. 5 moles of argon contain 30,110704285.10e23 molecules.
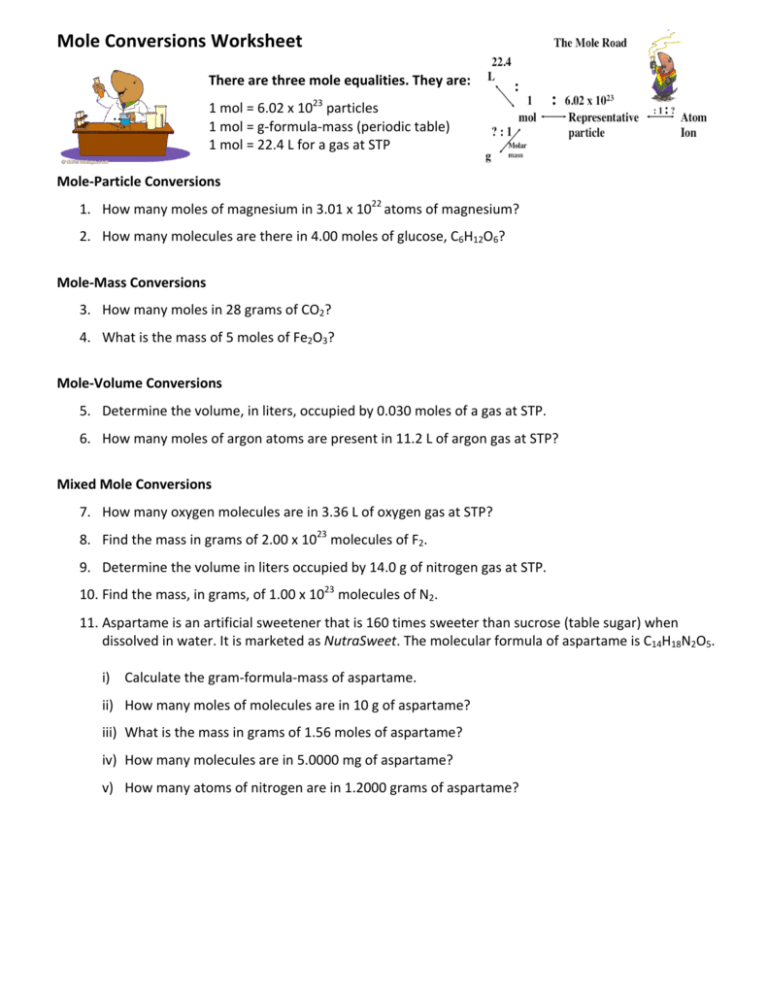 Source: studylib.net
Source: studylib.net
Grams × (1/39.948) = moles of argon. It is known that number of moles equal mass divided by molar mass. Here is the moles of argon to grams formula: Moles of argon × 39.948 = grams. 770 grams 3 how many moles are in 22 grams of argon.
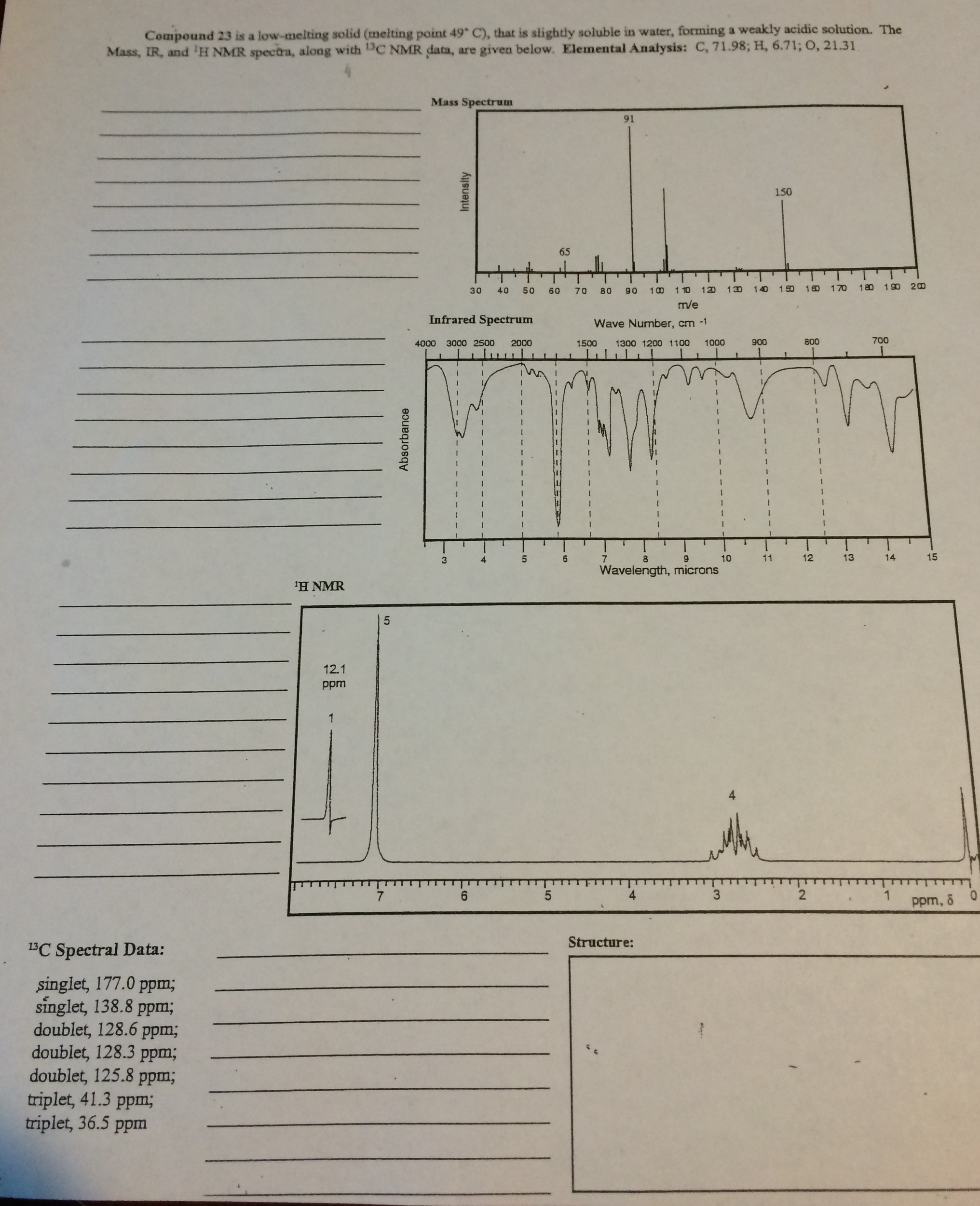 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
5 moles of argon contain 30,110704285.10e23 molecules. Therefore, to convert grams to moles of argon, we simply multiply grams by 1/39.948. Here is the moles of argon to grams formula: What is the number of moles in 22 grams of argon? How many molecules are in 5 moles of argon?

Therefore, number of moles =. It is known that number of moles equal mass divided by molar mass. How many grams are in 881 moles of magnesium. How many moles are there in 22 grams of argon? 22 grams of argon is equivalent to 0,55 moles.
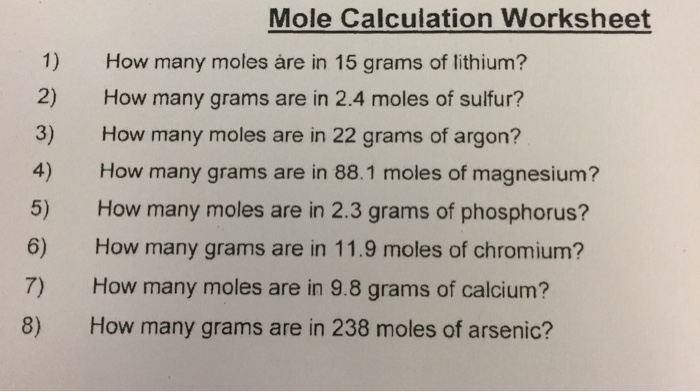
There are 05507 moles in 22 grams of argon. How.many moles are in 22 grams of argon? The si base unit for amount of substance is the mole. How many moles are in 22 grams of argon? Molecular weight of argon or mol the molecular formula for argon is ar.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
How many moles are there in 22 grams of argon? How many grams is 5.00 atoms of iron? How many molecules are in 5 moles of argon? 22 grams of argon is equivalent to 0,55 moles. Since, it is given that mass is 22 grams and molar mass of argon is 40 g/mol.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
It means that one mole of argon weighs 40g. It means that one mole of argon weighs 40g. How many moles are in 22 grams of carbon? What is the number of moles in 22 grams of argon? Be carefully when the calculations are related to noble gases.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
What is the number of moles in 22 grams of argon? How many molecules are in 5 moles of argon? There are 05507 moles in 22 grams of argon. More importantly for the purposes of making our converter, the atomic mass of argon is 39.948. Based on the formula above, we created the moles of argon to grams converter below.
 Source: coursehero.com
Source: coursehero.com
Mathematically, number of moles =. Since, it is given that mass is 22 grams and molar mass of argon is 40 g/mol. Molecular weight of argon or mol the molecular formula for argon is ar. How many moles are there in 22 grams of argon? The si base unit for amount of substance is the mole.
 Source: slideshare.net
Source: slideshare.net
You can view more details on each measurement unit: Can you please help me with number 7. Here is the moles of argon to grams formula: How many moles of argon are in 3.995 grams of argon gas? The si base unit for amount of substance is the mole.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
That means that one mole of argon weighs 39.948 grams (39.948 g/mol). 055 moles 4 how many grams are in 881 moles of magnesium. How many moles are in 22 grams of argon? Since, it is given that mass is 22 grams and molar mass of argon is 40 g/mol. Therefore, to convert grams to moles of argon, we simply multiply grams by 1/39.948.
 Source: argonnekonba.blogspot.com
Source: argonnekonba.blogspot.com
You can view more details on each measurement unit: 055 moles 4 how many grams are in 881 moles of magnesium. That means that one mole of argon weighs 39.948 grams (39.948 g/mol). Can you please help me with number 7. The si base unit for amount of substance is the mole.
 Source: afonsoquintaa.blogspot.com
Source: afonsoquintaa.blogspot.com
Here is the grams to moles of argon formula: How many moles are there in 22 grams of argon? The si base unit for amount of substance is the mole. We assume you are converting between grams argon and mole. 5 moles of argon contain 30,110704285.10e23 molecules.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are in 22 grams of argon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






