How are ions made from neutral atoms
How Are Ions Made From Neutral Atoms. In this activity we will explore another variation that can take place—the loss and gain of electrons. Both atoms are electrically neutral at the beginning, so the electron that is going to be transferred isn�t acted on by electrostatic forces. And secondly, does this simple? Hence, ions are the species which have the deficiency of electrons or excess of electrons which makes them positively charged and negatively charged.
 How ions are formed (Cation vs Anion) Best Chemistry Blog Digital From mydigitalkemistry.com
How ions are formed (Cation vs Anion) Best Chemistry Blog Digital From mydigitalkemistry.com
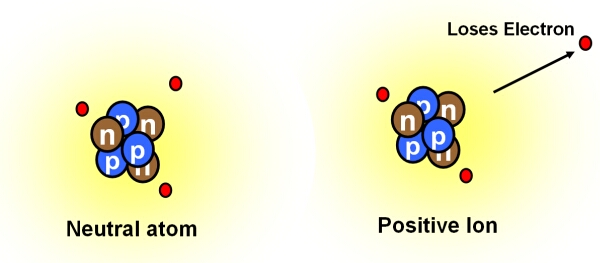
Positive and negative ions are formed by gaining or losing electrons from neutral atoms. Na loses an electron and gives it to cl because this makes both atoms more stable. Charged atoms are known as ions. I am, uh, in one, changes to the items nucleus or not. Ionization of neutral atoms compounds such as salts dissociate in solution into their ions, e.g., in solution sodium chloride exists as free na + and cl − ions. Ions are electrically charged particles produced by either removing electrons from a neutral a.
When a definite amount of energy called ionization energy is supplied to the atom, this loosely bound electron gets the energy needed to overcome nuclear attraction and thus it becomes free and.
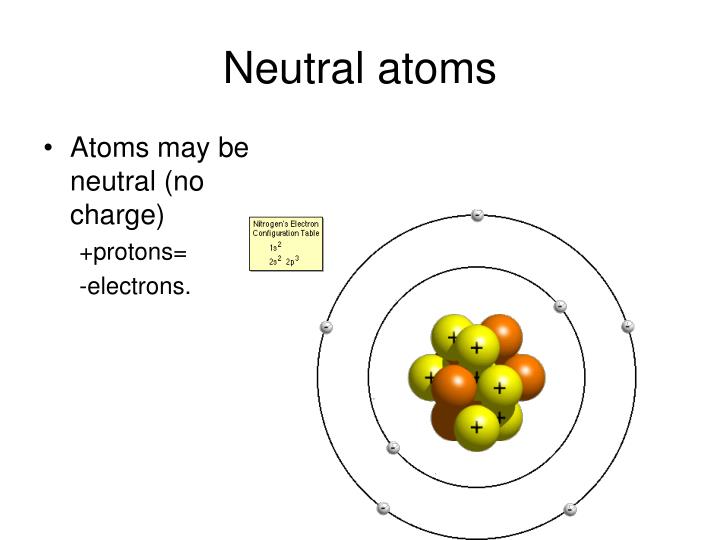
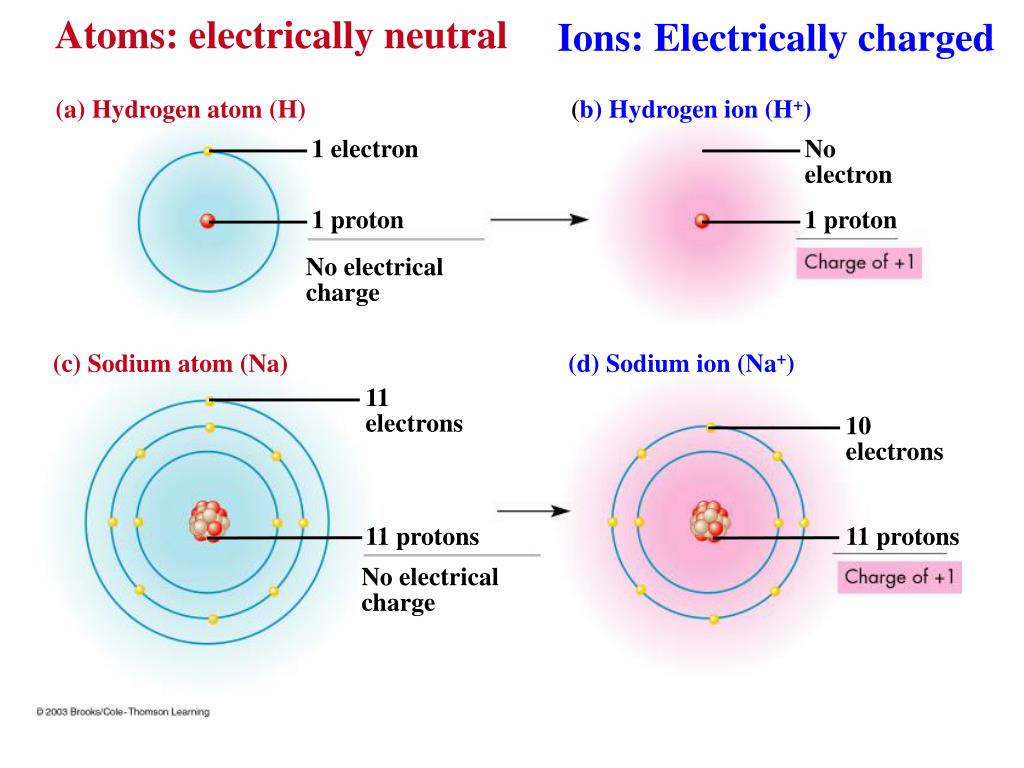
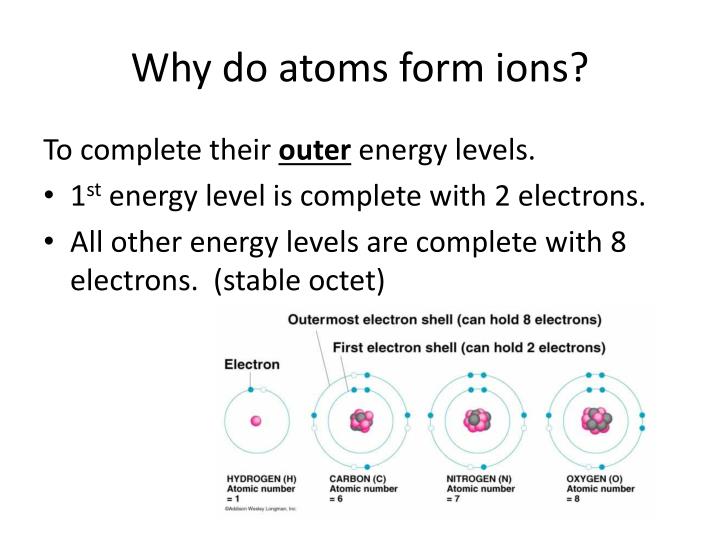
That is how i answer british, prominent them. Neutral atoms can be turned into positively charged. The process of gaining or losing electrons from a neutral atom or molecule, forming ions, is called ionization. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Pay attention to the word in bold: Examine the isotope symbols in model 1.
 Source: aakash.ac.in
Both atoms are electrically neutral at the beginning, so the electron that is going to be transferred isn�t acted on by electrostatic forces. The charge on 1 gram of a l 3 + ions is (e=electronic charge): Metallic elements produce positively charged ions by losing electrons while nonmetallic elements produce negatively charged ions by gaining electrons. An atom becomes an anion by gaining more electrons, so becoming negatively charged. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
 Source: sciencenotes.org
Source: sciencenotes.org
By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Nonmetal in the atom in the atom in the atom ion and the ion? By removing one or more electrons, neutral atoms can be converted into positively charged ions. Compounds that contain dissociable protons, or hydrogen ions, h + , or basic ions such as hydroxide ion, oh − , make acidic or basic solutions when they dissociate in water (see. Metallic elements produce positively charged ions by losing electrons while nonmetallic elements produce negatively charged ions by gaining electrons.
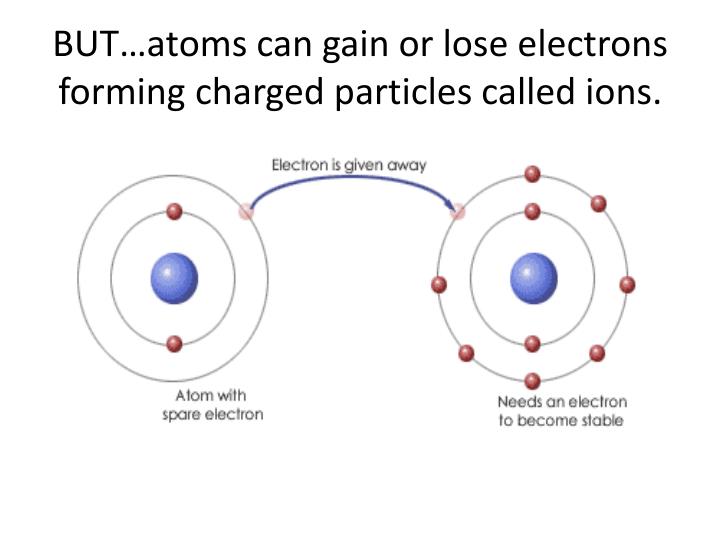 Source: slideserve.com
Source: slideserve.com
Both loss and gain of electrons. There is greater stabilisation achieved from the solvation of two na+ ions than if it existed as a neutral atom. So in answer to the first question, you know, basically, i answer child species when i n carries a positive charge. Circle choice protons electrons 3. Ions how are ions made from neutral atoms get the answers you need, now!
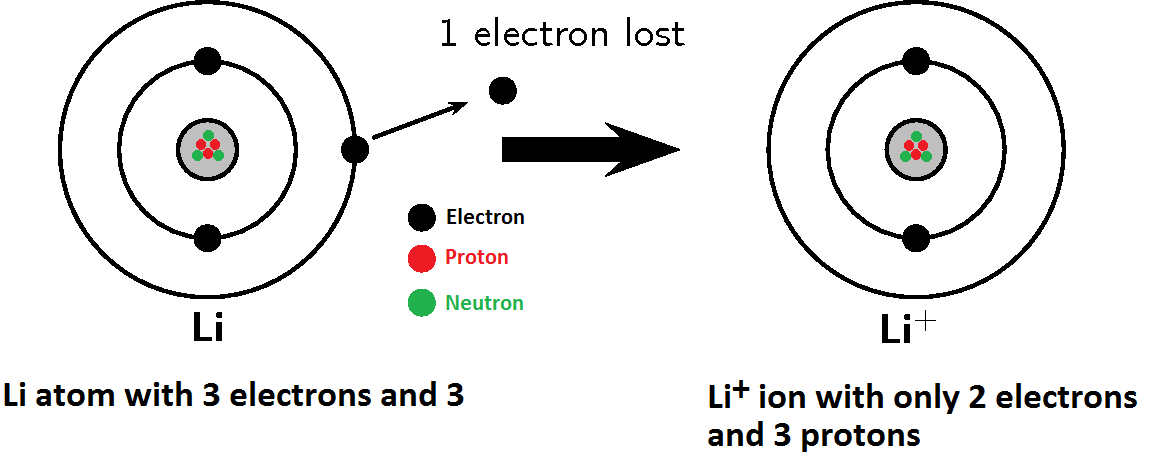 Source: basichemistry.blogspot.com
Source: basichemistry.blogspot.com
In this activity we will explore another variation that can take place—the loss and gain of electrons. The exchange of electrons between atoms is a very common way for chemical. Charged atoms are known as ions. The process of gaining or losing electrons from a neutral atom or molecule, forming ions, is called ionization. In this activity we will explore another variation that can take place—the loss and gain of electrons.
 Source: wtbblue.com
Source: wtbblue.com
Ionization in neutral atoms, the number of protons is equal to the number of electrons. Correct option is d) ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions. The exchange of electrons between atoms is a very common way for chemical. Ions how are ions made from neutral atoms? In metals, the electrons in the outermost shell are loosely bound to the nucleus.
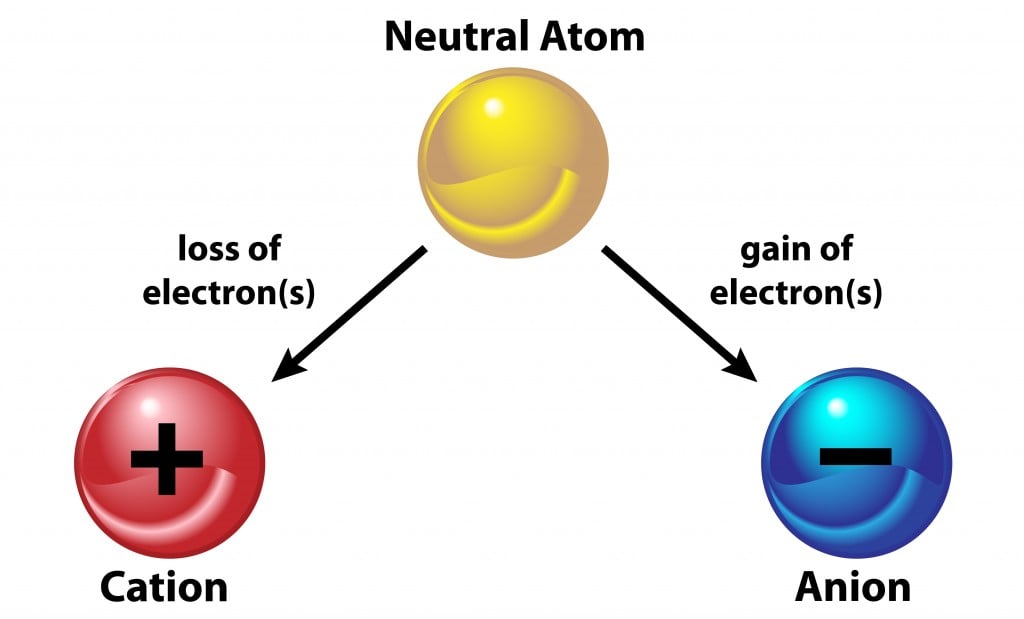 Source: scienceabc.com
Source: scienceabc.com
When an ion is formed, the number of protons does not change. By removing one or more electrons, neutral atoms can be converted into positively charged ions. In this activity we will explore another variation that can take place—the loss and gain of electrons. Ions how are ions made from neutral atoms? When the atom loses 1 electron, then the atom is converted into a cation having +1 charge (${{m}^{+}}$ ).
 Source: slideserve.com
Source: slideserve.com
Ions how are ions made from neutral atoms? Both loss and gain of electrons. Circle choice protons electrons 3. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Neutral atoms contain the same number of protons as electrons.
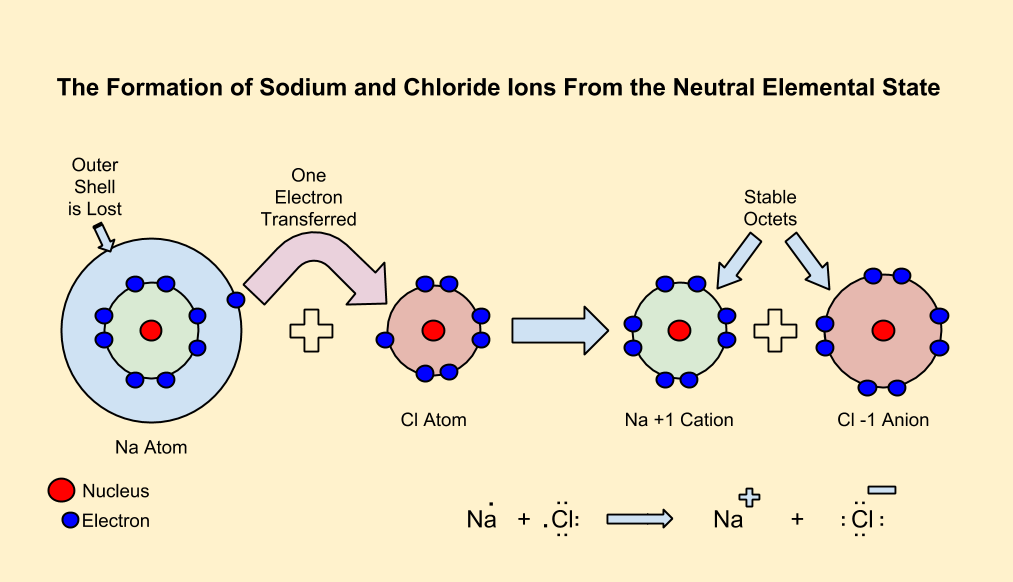 Source: socratic.org
Source: socratic.org
Both loss and gain of electrons. Neutral atoms can be turned into positively charged. Ions are electrically charged particles produced by either removing electrons from a neutral a. Examine the isotope symbols in model 1. The exchange of electrons between atoms is a very common way for chemical.
 Source: astronomy.swin.edu.au
Source: astronomy.swin.edu.au
So, the correct answer is ions are formed when a neutral atom gains or loses electrons to become positively or negatively. Na loses an electron and gives it to cl because this makes both atoms more stable. In metals, the electrons in the outermost shell are loosely bound to the nucleus. Neutral atom vs charged atom. Without such stabilising forces, you are right to think that ions are highly.
 Source: slideserve.com
Source: slideserve.com
Examine the isotope symbols in model 1. Ions are formed from neutral atoms by : Ions how are ions made from neutral atoms get the answers you need, now! Circle choice protons electrons 3. Similarly, when the atom loses 2 electrons, then the atom is converted into a cation having +2 charge (${{m}^{2+}}$).
 Source: sugarfunkdesign.blogspot.com
Source: sugarfunkdesign.blogspot.com
By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Hence, ions are the species which have the deficiency of electrons or excess of electrons which makes them positively charged and negatively charged. Positive and negative ions are formed by gaining or losing electrons from neutral atoms. In this activity we will explore another variation that can take place—the loss and gain of electrons. Charged atoms are known as ions.
 Source: thinkbiggerdesigns.blogspot.com
Source: thinkbiggerdesigns.blogspot.com
An atom is an electrically neutral particle with equal numbers of protons and electrons. Variation in the number of neutrons results in different isotopes of the element. There is greater stabilisation achieved from the solvation of two na+ ions than if it existed as a neutral atom. Nonmetal in the atom in the atom in the atom ion and the ion? Ions how are ions made from neutral atoms?
 Source: mydigitalkemistry.com
Source: mydigitalkemistry.com
Compounds that contain dissociable protons, or hydrogen ions, h + , or basic ions such as hydroxide ion, oh − , make acidic or basic solutions when they dissociate in water (see. So in answer to the first question, you know, basically, i answer child species when i n carries a positive charge. Hence, ions are the species which have the deficiency of electrons or excess of electrons which makes them positively charged and negatively charged. Ionization in neutral atoms, the number of protons is equal to the number of electrons. When an ion is formed, the number of protons does not change.
 Source: physics-and-radio-electronics.com
Source: physics-and-radio-electronics.com
Charged atoms are known as ions. Na loses an electron and gives it to cl because this makes both atoms more stable. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. When an ion is formed, the number of protons does not change. Both atoms are electrically neutral at the beginning, so the electron that is going to be transferred isn�t acted on by electrostatic forces.
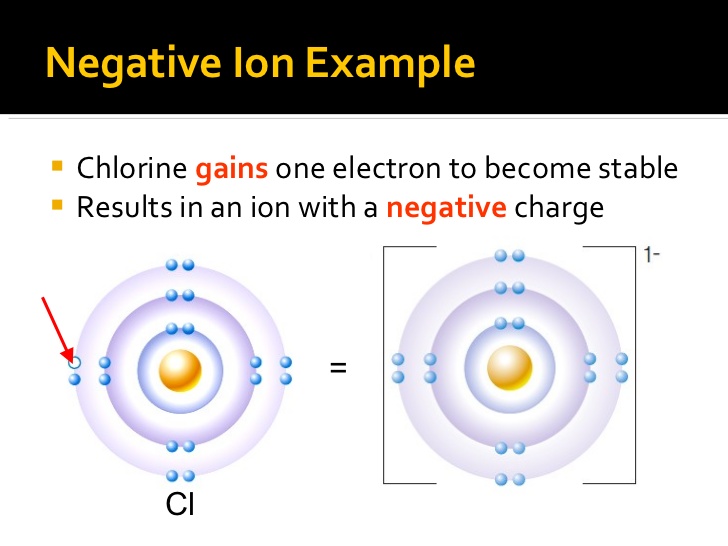 Source: socratic.org
Source: socratic.org
Ions are formed from neutral atoms by : The charge on 1 gram of a l 3 + ions is (e=electronic charge): Nonmetal in the atom in the atom in the atom ion and the ion? In metals, the electrons in the outermost shell are loosely bound to the nucleus. So, in this question, they asked.
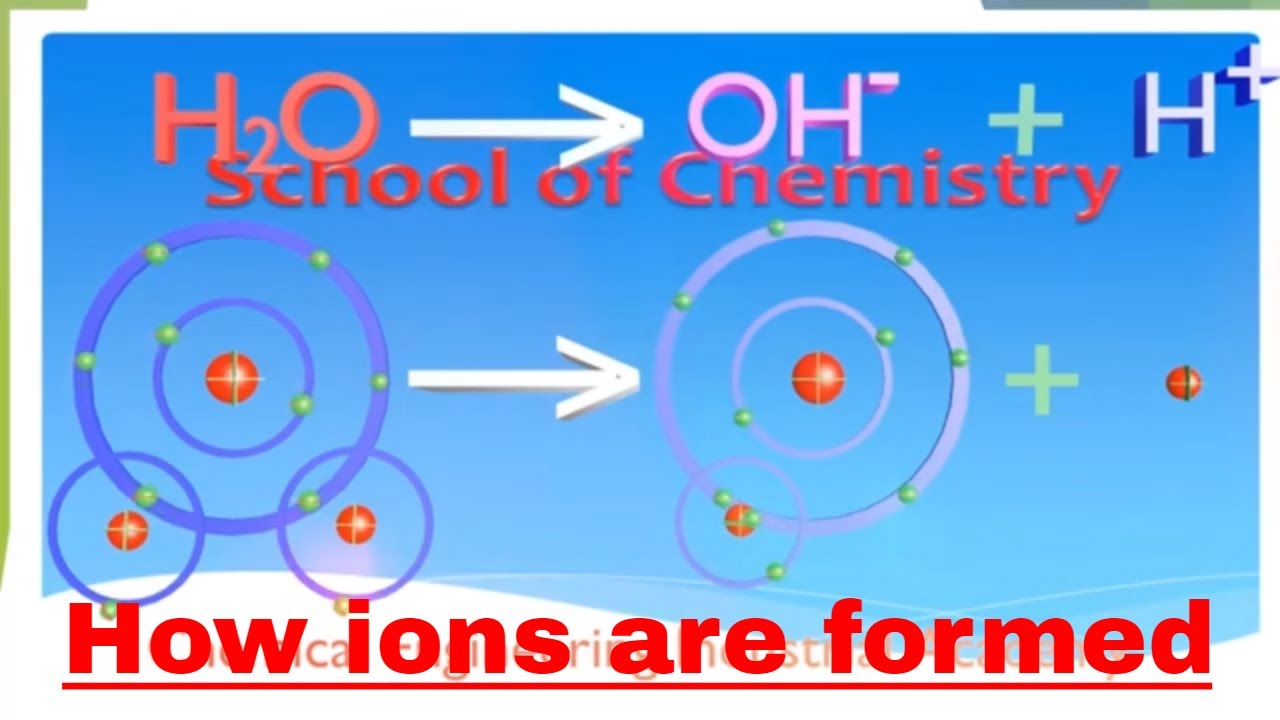 Source: youtube.com
Source: youtube.com
But what forces pulls the electron from na to cl? You have learned that not all atoms of an element are the same. Variation in the number of neutrons results in different isotopes of the element. An ion is formed when an electron is removed or added to an atom. Na loses an electron and gives it to cl because this makes both atoms more stable.
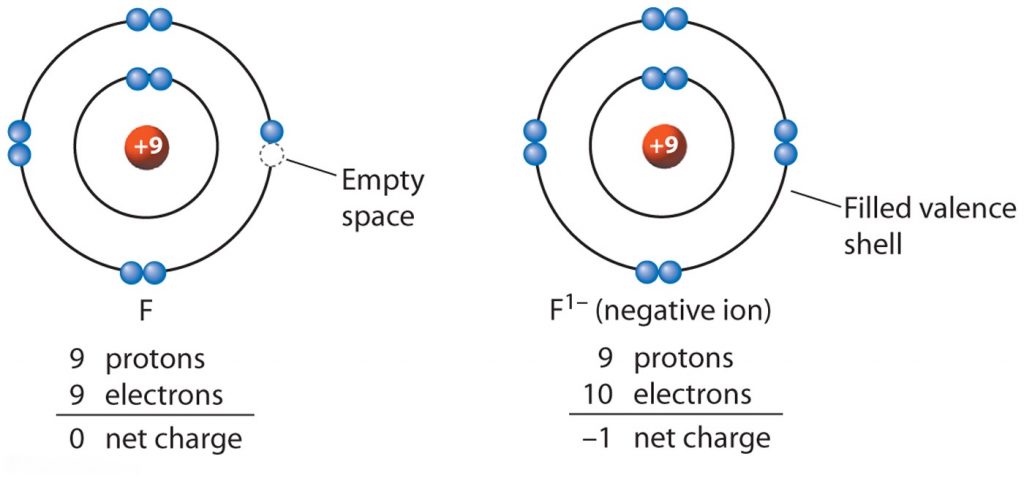 Source: kimcampion.com
Source: kimcampion.com
In this activity we will explore another variation that can take place—the loss and gain of electrons. Pay attention to the word in bold: Ions how are ions made from neutral atoms? An atom is an electrically neutral particle with equal numbers of protons and electrons. So, the correct answer is ions are formed when a neutral atom gains or loses electrons to become positively or negatively.
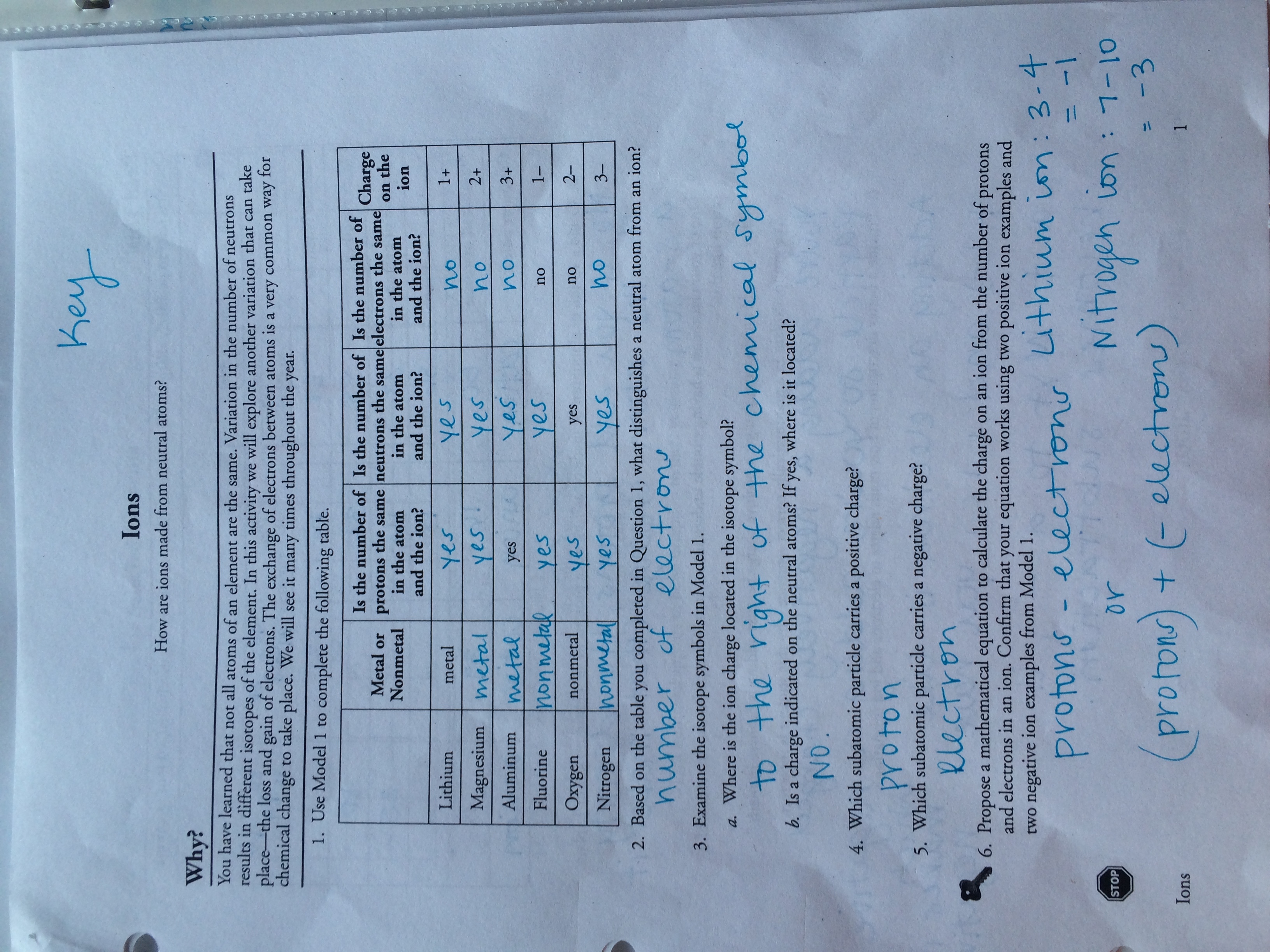 Source: nofisunthi.blogspot.com
Source: nofisunthi.blogspot.com
Ions how are ions made from neutral atoms? Correct option is d) ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions. So, the correct answer is ions are formed when a neutral atom gains or loses electrons to become positively or negatively. There is greater stabilisation achieved from the solvation of two na+ ions than if it existed as a neutral atom. Ionization in neutral atoms, the number of protons is equal to the number of electrons.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how are ions made from neutral atoms by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





