Freezing point depression examples in real life
Freezing Point Depression Examples In Real Life. Water) and a solute which you’ll mix with the solvent. A very common example of this phenomenon in everyday life is the salting of the roads in cold countries. The difference in temperature between the freezing point of the pure solvent and that of the solution is called the freezing point depression of a solution. The depression in the freezing point of a solution can be described by the following formula.
 Colligative Properties Worksheet From mychaume.com
Colligative Properties Worksheet From mychaume.com
Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug. Explains process of adding a solute to a solvent to affect the freezing point. Solutions freeze at lower temperatures than pure liquids. Depression is the most common mental illness associated with suicide or suicidal thoughts. K f = molal freezing point depression constant or cryoscopic constant in °c kg/mol. Many famous actors, actresses and other personalities battled depression.
It involves a solvent (e.g.
Solutions freeze at lower temperatures than pure liquids. The depression in the freezing point of a solution can be described by the following formula. What are the colligative properties? Brinicle, underwater icicle finger of death. It is a colligative property of solutions that is generally proportional to the molality of the added solute. M= δt f×w 11000×k f×w 2.
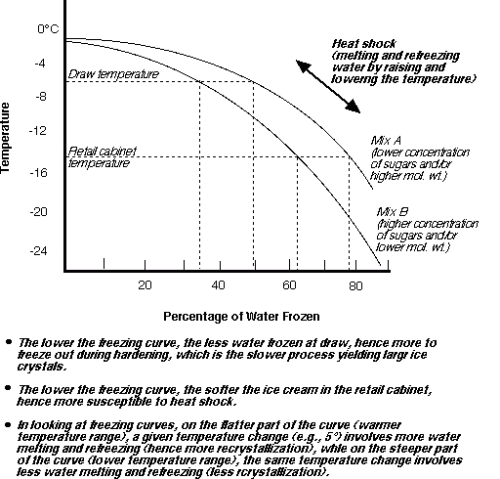 Source: uoguelph.ca
Source: uoguelph.ca
M= δt f×w 11000×k f×w 2. Examples of colligative properties include vapor pressure lowering, freezing point depression, osmotic pressure, and boiling point elevation.for example, adding a pinch of salt to a cup of water makes the water freeze at a lower temperature than it normally would, boil at a higher temperature, have a lower vapor pressure, and changes. Solutions freeze at lower temperatures than pure liquids. It involves a solvent (e.g. Pressure cooking is the most common method of cooking in almost every kitchen.
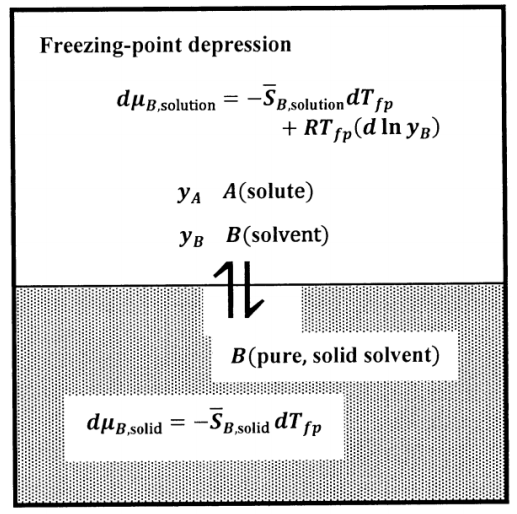 Source: chem.libretexts.org
Source: chem.libretexts.org
Δt = change in temperature in °c. Pure water freezes at 0°c. The difference in temperature between the freezing point of the pure solvent and that of the solution is called the freezing point depression of a solution. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes. M= δt f×w 11000×k f×w 2.
 Source: ck12.org
Source: ck12.org
This phenomenon is informally known as the icy brinicles of death because the supercooled brine solution formed also freezes any sea life that comes in contact with it as can be seen in the embedded bbc video below (run time = ~2 mins). Both the phases, i.e., solid and liquid, exist in equilibrium at the melting point. Explains process of adding a solute to a solvent to affect the freezing point. Examples of colligative properties include vapor pressure lowering, freezing point depression, osmotic pressure, and boiling point elevation.for example, adding a pinch of salt to a cup of water makes the water freeze at a lower temperature than it normally would, boil at a higher temperature, have a lower vapor pressure, and changes. How is freezing point depression used in real life?
 Source: ck12.org
Source: ck12.org
W 2= weight of solute. For example, water shows equilibrium at 0°c. The stuff you make yourself with real cream, and fruit, and custard is much better than any ice cream you can buy. How much energy is required to convert 100.g of ice at 0.00 °c to water vapor at 100.00 °c? Therefore, the melting point of a given solid is equal to the freezing point of a liquid.
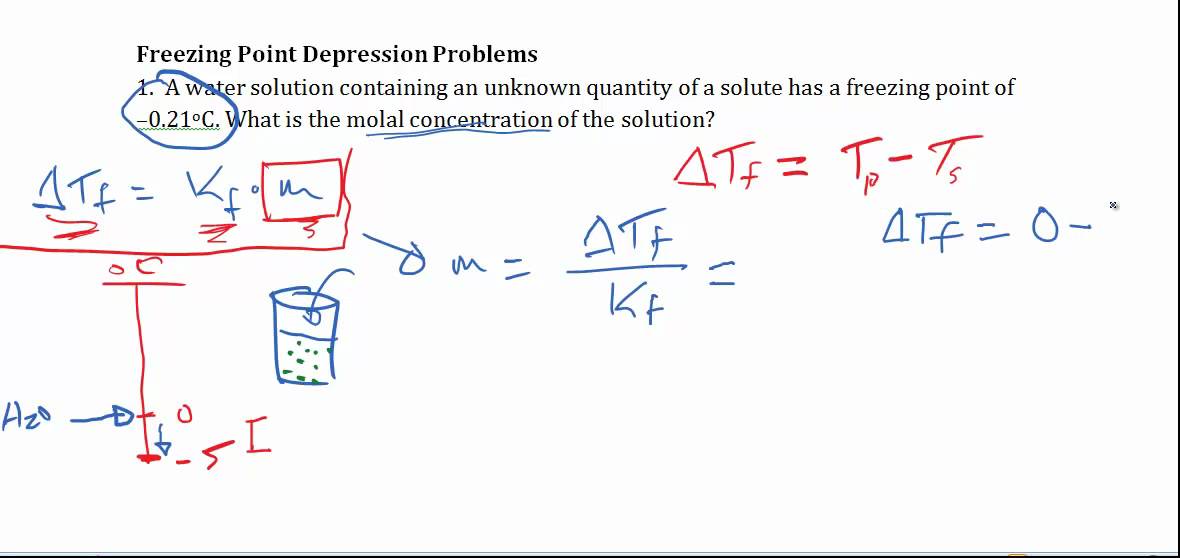 Source: youtube.com
Source: youtube.com
Explains process of adding a solute to a solvent to affect the freezing point. Both the phases, i.e., solid and liquid, exist in equilibrium at the melting point. So, if you have 5 moles of sugar (which equals 1711.5 grams of sugar in 1 kg of water) dissolved in water, we can determine the new freezing point as long as we know the freezing point of pure. Brinicle, underwater icicle finger of death. A very common example of this phenomenon in everyday life is the salting of the roads in cold countries.
 Source: chm2046l.weebly.com
Source: chm2046l.weebly.com
Seawater freezes at a lower temperature than. Solutions freeze at lower temperatures than pure liquids. How much energy is required to convert 100.g of ice at 0.00 °c to water vapor at 100.00 °c? Pressure cooking is the most common method of cooking in almost every kitchen. Pure water freezes at 0°c.
 Source: youtube.com
Source: youtube.com
In the laboratory, cryoscopy is sometimes used to estimate the molecular mass of a new substance, and this also relies on freezing point depression. Knowledge about brinicles is not novel. Water) and a solute which you’ll mix with the solvent. What are the colligative properties? Seawater freezes at a lower temperature than.
 Source: mychaume.com
Source: mychaume.com
So, if you have 5 moles of sugar (which equals 1711.5 grams of sugar in 1 kg of water) dissolved in water, we can determine the new freezing point as long as we know the freezing point of pure. Pure water freezes at 0°c. It involves a solvent (e.g. K f = molal freezing point depression constant or cryoscopic constant in °c kg/mol. Examples of colligative properties include vapor pressure lowering, freezing point depression, osmotic pressure, and boiling point elevation.for example, adding a pinch of salt to a cup of water makes the water freeze at a lower temperature than it normally would, boil at a higher temperature, have a lower vapor pressure, and changes.
 Source: youtube.com
Source: youtube.com
Therefore, the melting point of a given solid is equal to the freezing point of a liquid. The depression in the freezing point of a solution can be described by the following formula. Pressure cooking is the most common method of cooking in almost every kitchen. The stuff you make yourself with real cream, and fruit, and custard is much better than any ice cream you can buy. Knowledge about brinicles is not novel.
 Source: youtube.com
Source: youtube.com
Examples of colligative properties include vapor pressure lowering, freezing point depression, osmotic pressure, and boiling point elevation.for example, adding a pinch of salt to a cup of water makes the water freeze at a lower temperature than it normally would, boil at a higher temperature, have a lower vapor pressure, and changes. The depression in the freezing point of a solution can be described by the following formula. Δt = ik f m. For example, water shows equilibrium at 0°c. M= δt f×w 11000×k f×w 2.
 Source: toppr.com
Source: toppr.com
M= δt f×w 11000×k f×w 2. Seawater freezes at a lower temperature than. A very common example of this phenomenon in everyday life is the salting of the roads in cold countries. Knowledge about brinicles is not novel. Therefore, the melting point of a given solid is equal to the freezing point of a liquid.
![Freezing Point Depression [OpenStax] example 11.9 YouTube Freezing Point Depression [OpenStax] example 11.9 YouTube](https://i.ytimg.com/vi/82zASiUzyuc/maxresdefault.jpg) Source: youtube.com
Source: youtube.com
This phenomenon is informally known as the icy brinicles of death because the supercooled brine solution formed also freezes any sea life that comes in contact with it as can be seen in the embedded bbc video below (run time = ~2 mins). How is freezing point depression used in real life? Seawater freezes at a lower temperature than. Depression is the most common mental illness associated with suicide or suicidal thoughts. The stuff you make yourself with real cream, and fruit, and custard is much better than any ice cream you can buy.
 Source: chm2046l.weebly.com
Source: chm2046l.weebly.com
In the laboratory, cryoscopy is sometimes used to estimate the molecular mass of a new substance, and this also relies on freezing point depression. The difference in temperature between the freezing point of the pure solvent and that of the solution is called the freezing point depression of a solution. Solutions freeze at lower temperatures than pure liquids. Some emerged victorious while others lost the battle. Knowledge about brinicles is not novel.
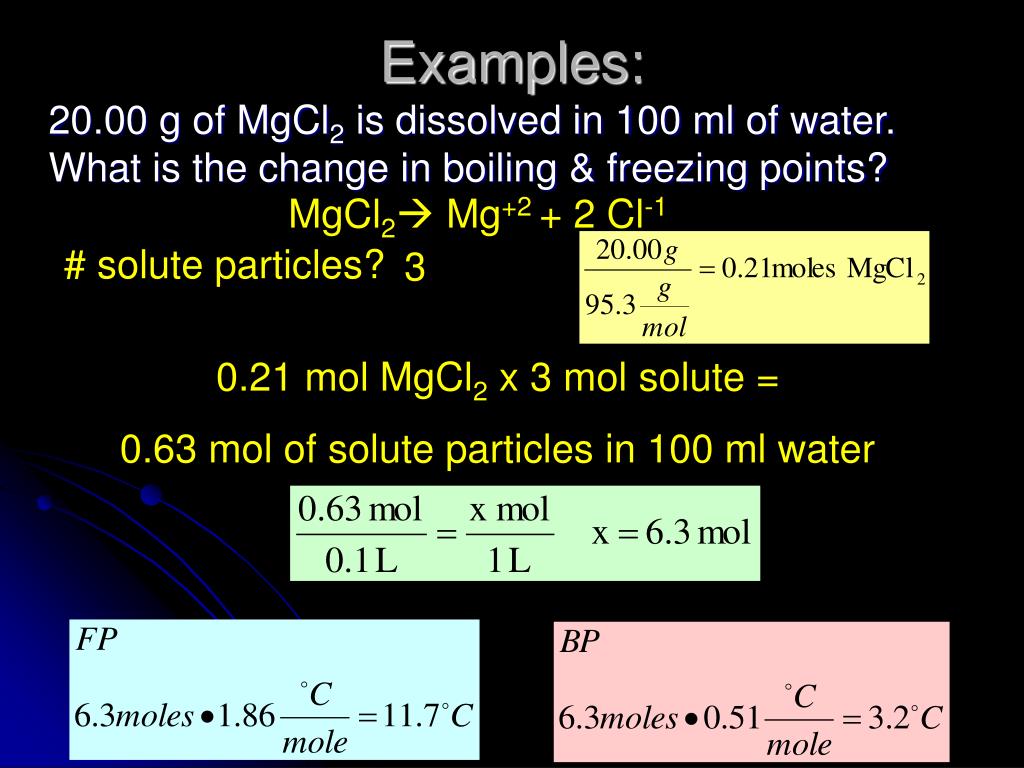 Source: slideserve.com
Source: slideserve.com
A very common example of this phenomenon in everyday life is salting of the roads in water. How is freezing point depression used in real life? So, if you have 5 moles of sugar (which equals 1711.5 grams of sugar in 1 kg of water) dissolved in water, we can determine the new freezing point as long as we know the freezing point of pure. Seawater freezes at a lower temperature than. Pure water freezes at 0°c.
 Source: youtube.com
Source: youtube.com
Inside a pressure cooker, the water is heated, and eventually it boils into steam. Brinicle, underwater icicle finger of death. K f = molal freezing point depression constant or cryoscopic constant in °c kg/mol. Solutions freeze at lower temperatures than pure liquids. Some emerged victorious while others lost the battle.
 Source: golifescience.com
Source: golifescience.com
How is freezing point depression used in real life? Pressure cooking is the most common method of cooking in almost every kitchen. Solutions freeze at lower temperatures than pure liquids. Depression is the most common mental illness associated with suicide or suicidal thoughts. The stuff you make yourself with real cream, and fruit, and custard is much better than any ice cream you can buy.
 Source: goodttorials.blogspot.com
Source: goodttorials.blogspot.com
Some emerged victorious while others lost the battle. Pure water freezes at 0°c. Δt = ik f m. Therefore, the melting point of a given solid is equal to the freezing point of a liquid. The freezing point depression says that the freezing point of a mixture of two components is lower than that of individual components.
 Source: youtube.com
Source: youtube.com
Pure water freezes at 0°c. K f = molal freezing point depression constant or cryoscopic constant in °c kg/mol. Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug. A very common example of this phenomenon in everyday life is the salting of the roads in cold countries. Freezing point depression refers to the lowering of the freezing point of solvents upon the addition of solutes.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title freezing point depression examples in real life by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






