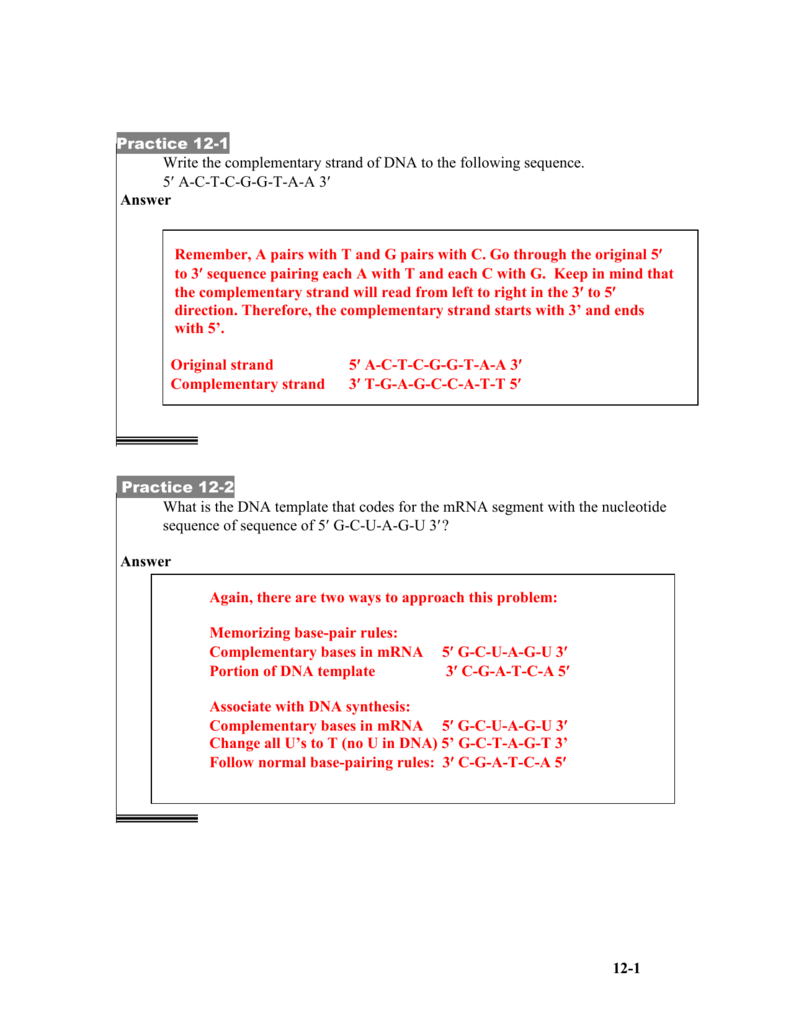
Chemistry flame test lab report
Chemistry Flame Test Lab Report. This activity is called a flame test and its a real procedure used in labs. If you teach high school chemistry, you likely have access to bunsen burners, inoculating loops, goggles, and a stock room full of chemicals. Group of mt che 009 flame test objective: Flame test for lab 3 flame test lab report introduction the electrons of an atom have.

Why do we see colors of the flame in the experiment. 2 (591 words) research report on china’s low smoke halogen free flame retardant polypropylene (pp) industry pages: Group of mt che 009 flame test objective: A photon in short is light. Atoms produce light by putting energy in, the electron then becomes excited and goes up an energy level, the electron then falls back down to its ground state, and out comes. To better understand these concepts and make chemistry more interactive, we did an experiment about compounds element light and wavelength.
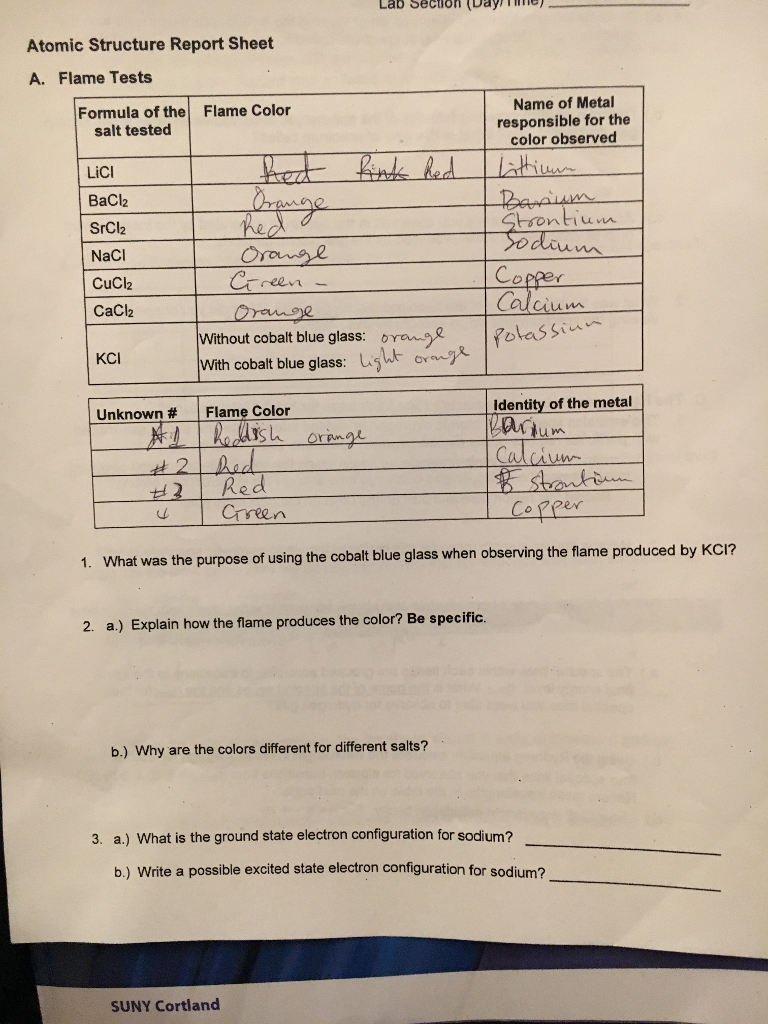
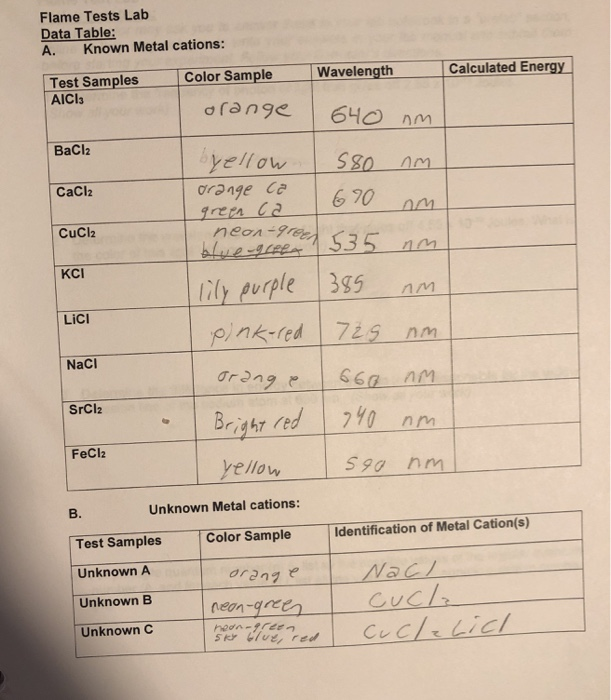
Each metal when under a flame gives off a unique color or radiation with frequencies in which the photoreceptors in our eyes can interpret.
2 (417 words) flame detection using cnn in safety surveillance pages: Table 2 shows that you can identify a metal in a compound by using a flame test. Then, place one of the saturated sticks into the flame. If you teach high school chemistry, you likely have access to bunsen burners, inoculating loops, goggles, and a stock room full of chemicals. Chemistry flame test lab report. The purpose of the flame test is to demonstrate to students the variety of colors produced when different metals or salts meet a flame.

Flame colors of metals pages: Table 2 shows that you can identify a metal in a compound by using a flame test. To determine the colours of the atomic emission spectra of several metallic ions by the flame test. The elements are a rainbow when they burn purpose the purpose of this experiment is observe different substances being exposed to high temperatures and see how they react. University our lady of fatima university;
 Source: slidesharetrick.blogspot.com
Source: slidesharetrick.blogspot.com
You will be removed from lab without them. This activity is called a flame test and its a real procedure used in labs. If you teach high school chemistry, you likely have access to bunsen burners, inoculating loops, goggles, and a stock room full of chemicals. A) perform flame tests of metal cations in order to observe their characteristic colors, b) match the flame colors observed to an appropriate wavelength of visible light, and then perform. 3 (713 words) projectile motion lab report:
 Source: coursehero.com
Source: coursehero.com
A) perform flame tests of metal cations in order to observe their characteristic colors, b) match the flame colors observed to an appropriate wavelength of visible light, and then perform. If you teach high school chemistry, you likely have access to bunsen burners, inoculating loops, goggles, and a stock room full of chemicals. Atoms produce light by putting energy in, the electron then becomes excited and goes up an energy level, the electron then falls back down to its ground state, and out comes. 2 (354 words) flame test lab pages: This activity is called a flame test and its a real procedure used in labs.

To do this, rinse it with distilled water from the wash bottle, dip it into the hydrochloric acid, and place it in the burner flame for a few moments. Flame tests are a quick method of producing the characteristics colours of metallic ions. The elements are a rainbow when they burn purpose the purpose of this experiment is observe different substances being exposed to high temperatures and see how they react. Below was my lab report for the experiment. 2 (564 words) laboratory report :
 Source: 2020edwardip.weebly.com
Source: 2020edwardip.weebly.com
7 (1936 words) stroop test lab report pages: Chemistry flame test lab report. The flame test is a safer version of the traditional rainbow demonstration, an exercise popularly conducted in chemistry classrooms. This happens because every metal has a different bright line spectra. Chemistry flame test lab report.

Lucius flame test lab ___ introduction the flame test is used to detect metal ions based on the elements on the element�s emission spectrum. Each metal when under a flame gives off a unique color or radiation with frequencies in which the photoreceptors in our eyes can interpret. Then, place one of the saturated sticks into the flame. To do this, rinse it with distilled water from the wash bottle, dip it into the hydrochloric acid, and place it in the burner flame for a few moments. 2 (564 words) laboratory report :
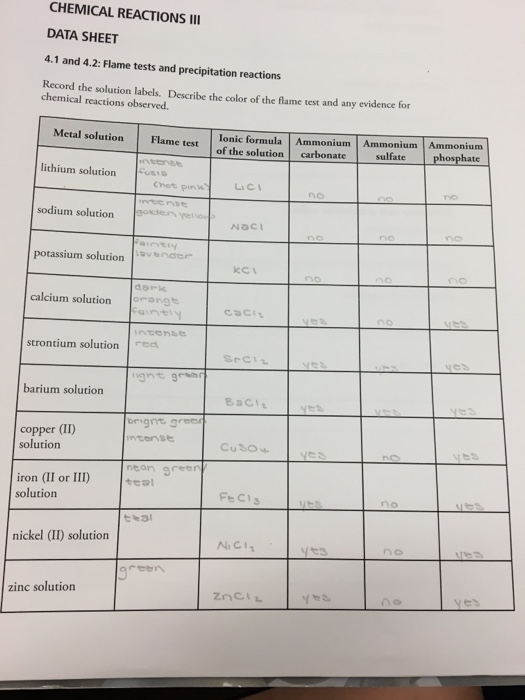 Source: slidesharetrick.blogspot.com
Source: slidesharetrick.blogspot.com
This happens because every metal has a different bright line spectra. Various elements burn with different colored flames. 7 (1936 words) stroop test lab report pages: Flame test for lab 3 flame test lab report introduction the electrons of an atom have. Flame test lab report general chemistry chm131 experiment no:
 Source: markedbyteachers.com
Source: markedbyteachers.com
Spectroscopy is the study of the electromagnetic radiation emitted or absorbed by the atoms and molecules. To determine the colours of the atomic emission spectra of several metallic ions by the flame test. Flame test lab report conclusion in the flame test lab i learned about how different metal ions produce different color flames when they are put under a burner flame. This activity is called a flame test and its a real procedure used in labs. Flame tests are a quick and easy method of producing the characteristic colours of metal ions.
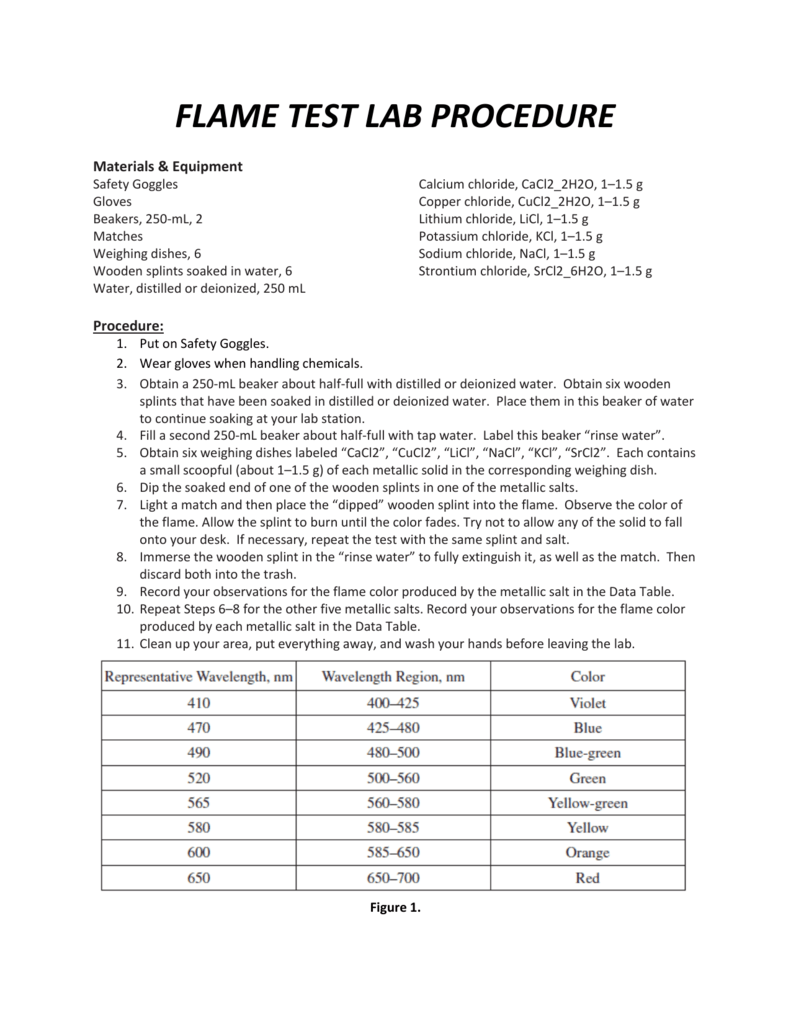 Source: studylib.net
Source: studylib.net
If you teach high school chemistry, you likely have access to bunsen burners, inoculating loops, goggles, and a stock room full of chemicals. Measurements length, volume and temperature name student id name results name of solution introducing ask an expert 🎉 we brought real experts onto our platform to help you even better! Atoms produce light by putting energy in, the electron then becomes excited and goes up an energy level, the electron then falls back down to its ground state, and out comes. Each metal when under a flame gives off a unique color or radiation with frequencies in which the photoreceptors in our eyes can interpret. The metal in the compound was identified by the light orange color seen during the flame test.

The purpose of this lab is to investigate atomic absorption and emission spectroscopy through the use of flame tests. Due to the emission spectrum of the element the compound turned the flame a certain color. With the metal under the flame it produces. Flame test lab report pages: 2 (564 words) laboratory report :

A) perform flame tests of metal cations in order to observe their characteristic colors, b) match the flame colors observed to an appropriate wavelength of visible light, and then perform. 4 (967 words) chemistry lab experiment: The flame emits a color because each element has an exactly defined emission spectrum, which one can use to identify them. Each metal when under a flame gives off a unique color or radiation with frequencies in which the photoreceptors in our eyes can interpret. The color is a combination of wavelengths of each transition and.

The objectives of this lab are to: Various elements burn with different colored flames. Lucius flame test lab ___ introduction the flame test is used to detect metal ions based on the elements on the element�s emission spectrum. 4 (967 words) chemistry lab experiment: 2 (354 words) flame test lab pages:
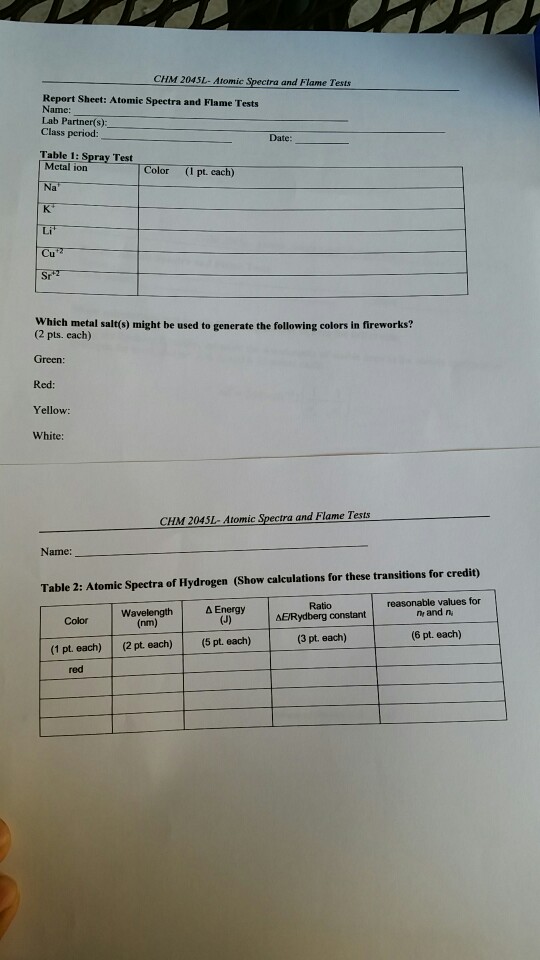
To do this, rinse it with distilled water from the wash bottle, dip it into the hydrochloric acid, and place it in the burner flame for a few moments. Then, make a chart identifying the color emissions of the ten different compounds previously observed in the laboratory. Table 1 proves my hypothesis to be correct. 6 (1654 words) umberto eco’s novel the mysterious flame of queen. To determine the colours of the atomic emission spectra of several metallic ions by the flame test.
 Source: slidesharetrick.blogspot.com
Source: slidesharetrick.blogspot.com
Why do we see colors of the flame in the experiment. Due to the emission spectrum of the element the compound turned the flame a certain color. Chemistry flame test lab report pages: Measurements length, volume and temperature name student id name results name of solution introducing ask an expert 🎉 we brought real experts onto our platform to help you even better! Group of mt che 009 flame test objective:
 Source: slidesharetrick.blogspot.com
Source: slidesharetrick.blogspot.com
The elements are a rainbow when they burn purpose the purpose of this experiment is observe different substances being exposed to high temperatures and see how they react. 2 (591 words) research report on china’s low smoke halogen free flame retardant polypropylene (pp) industry pages: Each metal when under a flame gives off a unique color or radiation with frequencies in which the photoreceptors in our eyes can interpret. Measurements length, volume and temperature name student id name results name of solution introducing ask an expert 🎉 we brought real experts onto our platform to help you even better! Flame test lab report pages:
 Source: slidesharetrick.blogspot.com
Source: slidesharetrick.blogspot.com
The purpose of this lab is to investigate atomic absorption and emission spectroscopy through the use of flame tests. Metal salts contain loosely held electrons that are easily exited on heating. Lucius flame test lab ___ introduction the flame test is used to detect metal ions based on the elements on the element�s emission spectrum. 2 (591 words) research report on china’s low smoke halogen free flame retardant polypropylene (pp) industry pages: The metal in the compound was identified by the light orange color seen during the flame test.
 Source: studylib.net
Source: studylib.net
Then, place one of the saturated sticks into the flame. The emission of energy in the visible portion of the spectrum as those electrons return to lower energy levels produces a coloured flame. 2 (591 words) research report on china’s low smoke halogen free flame retardant polypropylene (pp) industry pages: Metal salts contain loosely held electrons that are easily exited on heating. The elements are a rainbow when they burn purpose the purpose of this experiment is observe different substances being exposed to high temperatures and see how they react.
 Source: studocu.com
Source: studocu.com
Chemistry flame test lab report. This is the “go big or go home!” option. Metal salts contain loosely held electrons that are easily exited on heating. Light the bunsen burner, and adjust it so that you have a small (2 cm) blue cone of flame. 4 (967 words) chemistry lab experiment:
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title chemistry flame test lab report by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.