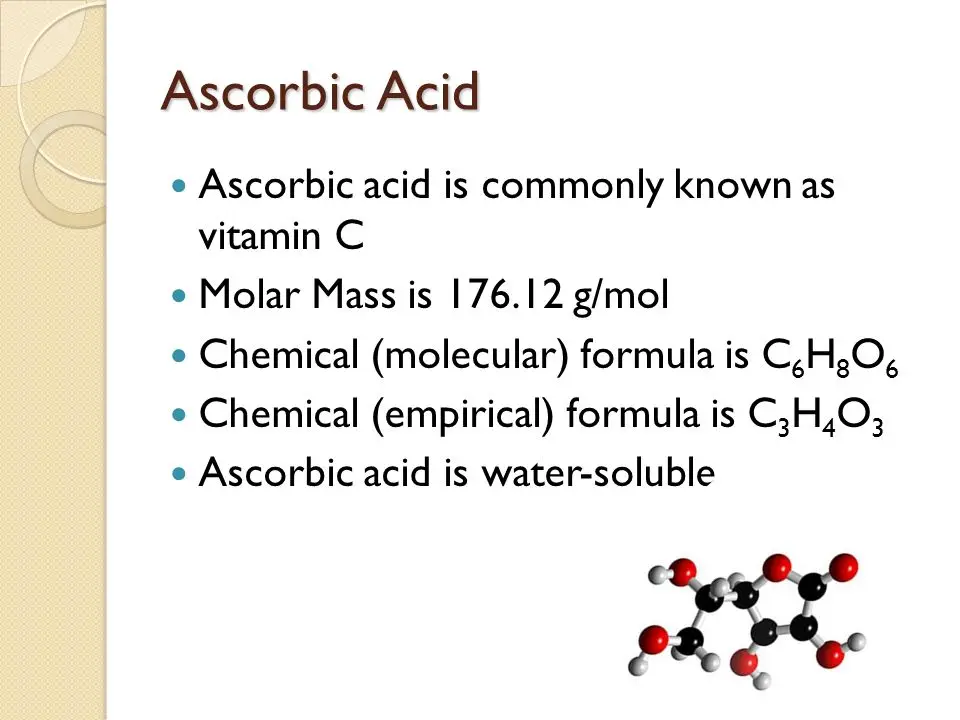
Ascorbic acid empirical formula
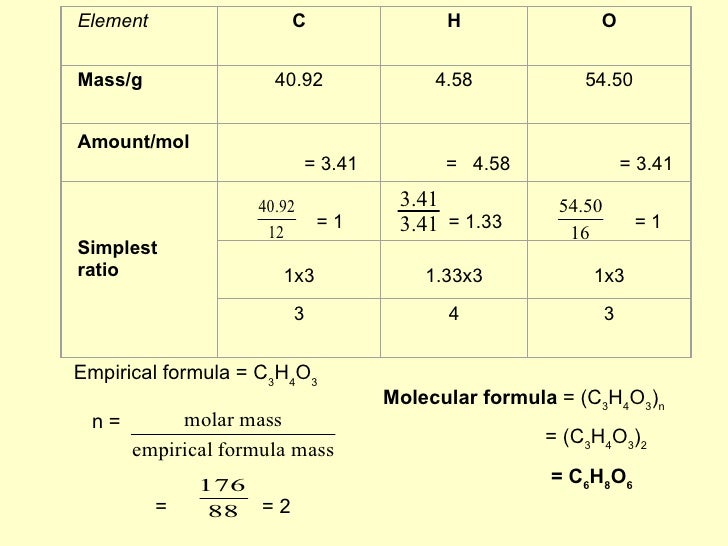
Ascorbic Acid Empirical Formula. If carbon is 4091 hydrogen is 45 and oxygen is 5450. 54.5 / 16 = 3.40625 mol / 3.40625 = 1 now, as you understand that the subscripts in a formula must be whole. Ascorbic acid is an organic compound with formula c 6 h 8 o 6, originally called hexuronic acid.it is a white solid, but impure samples can appear yellowish. Determine the empirical formula of ascorbic acid.
 Bulk Sale Vitamin C ( Ascorbic Acid ) For Food/pharmaceutical Industry From alibaba.com
Bulk Sale Vitamin C ( Ascorbic Acid ) For Food/pharmaceutical Industry From alibaba.com
Determine the empirical formula of ascorbic acid if it is composed of 40.92% carbon, 4. It is a mild reducing agent. The empirical formula of ascorbic acid. C + 4 x atomic wt h + 3 x atomic weight o empirical weight = 3 x 12 + 4 x 1 + 3 x 16 =88 so, mutiplier = 176.12g / 88g =. C 6 h 6 mg 1.5 o 9 p · xh 2 o. The molecular formula of ascorbic acid is c6 h8 o6.
Hence it is also called as ethenyl group at times.
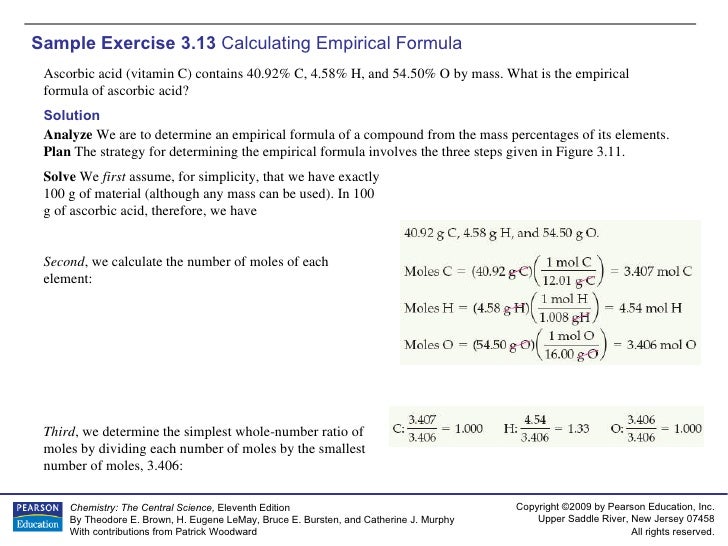
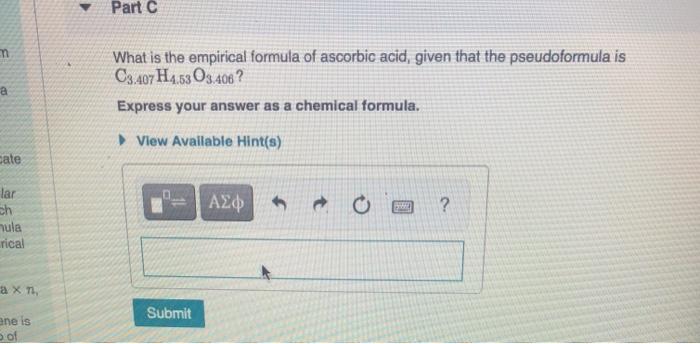
What is the empirical formula of ascorbic acid given that the pseudoformula is c3.407h4.53o3.406? The chemical or molecular formula of ascorbic acid is c 6 h 8 o 6. Determine the empirical formula of ascorbic acid. A solution is 0.10 m in ascorbic acid and 0.20 m in sodium ascorbate, nahc6h6o6. 289.54 (anhydrous basis) compare product no. It acts as an antioxidant and is widely used in food additives.
 Source: chegg.com
Source: chegg.com
C 6 h 6 mg 1.5 o 9 p · xh 2 o. Yes, ascorbic acid is nothing else but vitamin c only. Vitamin c is known chemically by the name ascorbic acid determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen. The empirical formula of ascorbic acid can be described as the simplified form of the chemical formula of ascorbic acid where the ratio of each atom is still observed. Is ascorbic acid just as vitamin c?
 Source: slideshare.net
Source: slideshare.net
The molar mass of ascorbic acid is 176.1 g/mol. The empirical formula of ascorbic acid can be described as the simplified form of the chemical formula of ascorbic acid where the ratio of each atom is still observed. 4.58 / 1 = 4.58 mol / 3.40625 = 1.345 ~ ??? The molecular formula of ascorbic acid is c6 h8 o6. Ascorbic acid is an organic compound with formula c 6 h 8 o 6, originally called hexuronic acid.it is a white solid, but impure samples can appear yellowish.
 Source: chegg.com
Source: chegg.com
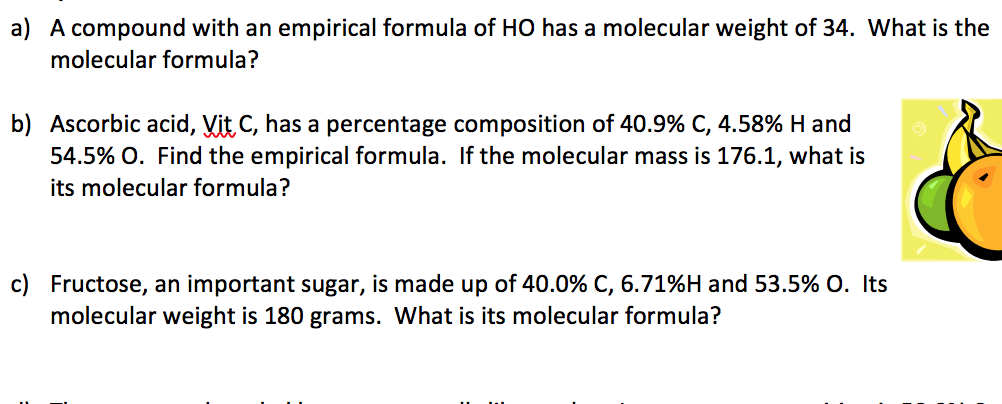
Question ascorbic acid (vitamin c) is a diprotic acid that has the formula h2c6h6o6. Find ascorbic acid and related products for scientific research at merck. Yes, ascorbic acid is nothing else but vitamin c only. Why is ascorbic acid bad in excess amounts? A 10.000 g sample of ascorbic acid was analyzed and found to contain 4.091 g of carbon, 0.458 g of hydrogen and the remainder is oxygen.
 Source: arwenmartinez.blogspot.com
Source: arwenmartinez.blogspot.com
Want to see the full answer? In its solid form, it is white to pale yellow compound and easily dissolves in water. Ascorbic acid belongs to the monosaccharide family and has a chemical formula c 6 h 8 o 6. The empirical formula of ascorbic acid. 0.1 pts question 19 vitamin c is known chemically by the name ascorbic acid.
 Source: alibaba.com
Source: alibaba.com
Mass of 78.1 g/mol with an empirical formula of glucose is ch2o [ c6h12o6 ] of a that. The molecular formula of ascorbic acid is c6 h8 o6. The empirical formula of ascorbic acid can be described as the simplified form of the chemical formula of ascorbic acid where the ratio of each atom is still observed. The empirical formula of ascorbic acid. 4.58 / 1 = 4.58 mol / 3.40625 = 1.345 ~ ???
 Source: slideserve.com
Source: slideserve.com
Why is ascorbic acid bad in excess amounts? C + 4 x atomic wt h + 3 x atomic weight o empirical weight = 3 x 12 + 4 x 1 + 3 x 16 =88 so, mutiplier = 176.12g / 88g =. Also 1:1, so the empirical formula of a compound is a representation of the simplest whole number ratio the. 4.57% h, and 54.50% 0? 40.92 / 12 = 3.41 mol / 3.40625 = 1.001 ~ 1 * h:
 Source: wordpress-778085-2806436.cloudwaysapps.com
Source: wordpress-778085-2806436.cloudwaysapps.com
C 6 h 6 mg 1.5 o 9 p · xh 2 o. Determine the empirical formula of ascorbic acid. Find ascorbic acid and related products for scientific research at merck. Mol o, the empirical formula is c3h4o3.2 apr 2022 what is the empirical molar mass of ascorbic acid? 0.1 pts question 19 vitamin c is known chemically by the name ascorbic acid.
 Source: bartleby.com
Source: bartleby.com
The empirical formula of ascorbic acid can be described as the simplified form of the chemical formula of ascorbic acid where the ratio of each atom is still observed. If carbon is 4091 hydrogen is 45 and oxygen is 5450. Vitamin c (ascorbic acid) is a key vitamin for animals and plants. Determine the empirical formula of ascorbic acid if it is composed of 40.92% carbon, 4.58% hydrogen, and 54.50% oxygen och 0 c₂h₂o2 none of these choices o c3h2o3 cho ; C + 4 x atomic wt h + 3 x atomic weight o empirical weight = 3 x 12 + 4 x 1 + 3 x 16 =88 so, mutiplier = 176.12g / 88g =.
The experimentally determined molecular mass […] 4.58 / 1 = 4.58 mol / 3.40625 = 1.345 ~ ??? In this jc1 webinar let�s learn how to calculate empirical and molecular formula of ascorbic acid or vitamin c.the empirical formula of a compound shows the. Because one mole of atoms of any. Find ascorbic acid and related products for scientific research at merck.

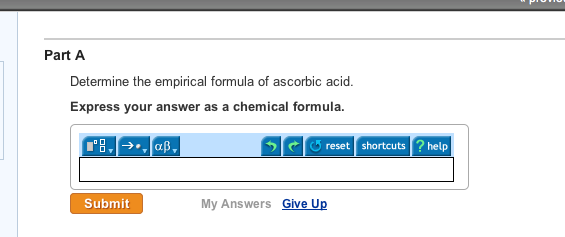
A sample of the vitamin supplement was analyzed by titrating a 0.100. Express answer as a chemical formula. Question ascorbic acid (vitamin c), h2c6h6o6, has a pka of 4.10. Ascorbic acid belongs to the monosaccharide family and has a chemical formula c 6 h 8 o 6. Is ascorbic acid just as vitamin c?
 Source: chegg.com
Source: chegg.com
It dissolves well in water to give mildly acidic solutions. Find ascorbic acid and related products for scientific research at merck. Determine the empirical formula of ascorbic acid if it is composed of 40.92% carbon, 4.58% hydrogen, and 54.50% oxygen och 0 c₂h₂o2 none of these choices o c3h2o3 cho ; Vitamin c (ascorbic acid) is a key vitamin for animals and plants. It dissolves well in water to give mildly acidic solutions.
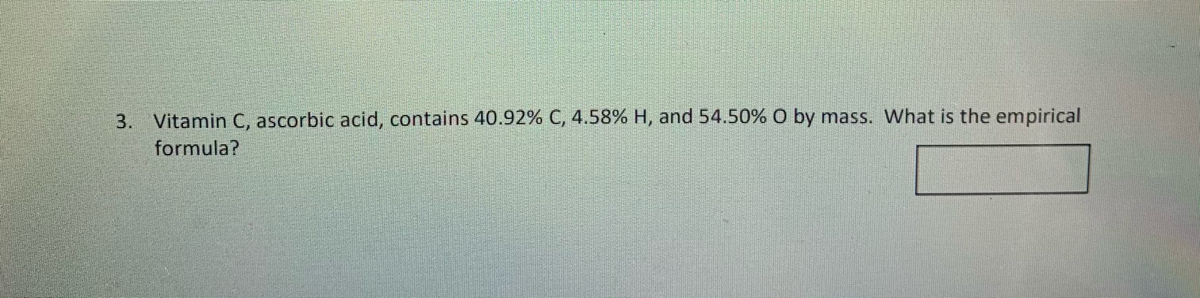 Source: bartleby.com
Source: bartleby.com
Determine the molecular formula of ascorbic acid. A solution is 0.10 m in ascorbic acid and 0.20 m in sodium ascorbate, nahc6h6o6. C 6 h 6 mg 1.5 o 9 p · xh 2 o. Hence it is also called as ethenyl group at times. Vitamin c is known chemically by the name ascorbic acid determine the empirical formula of ascorbic acid if it is composed of 4092 carbon 458 hydrogen and 5450 oxygen.
 Source: chegg.com
Source: chegg.com
Question ascorbic acid (vitamin c) is a diprotic acid that has the formula h2c6h6o6. A sample of the vitamin supplement was analyzed by titrating a 0.100. In its solid form, it is white to pale yellow compound and easily dissolves in water. Question ascorbic acid (vitamin c), h2c6h6o6, has a pka of 4.10. 4.57% h, and 54.50% 0?
 Source: chegg.com
Source: chegg.com
It is a mildly acidic solution. Vitamin c (ascorbic acid) is a key vitamin for animals and plants. Be produced or 13 g/mol is its empirical formula for benzene is c4h8 0.480 g carbon and 0.640 g…. In this jc1 webinar let�s learn how to calculate empirical and molecular formula of ascorbic acid or vitamin c.the empirical formula of a compound shows the. Mol o, the empirical formula is c3h4o3.2 apr 2022 what is the empirical molar mass of ascorbic acid?
The empirical formula of ascorbic acid. The empirical formula of ascorbic acid. Ascorbic acid, if taken in large amounts, may prove to be toxic to our body. The molar mass of ascorbic acid is 176.1 g/mol. Express answer as a chemical formula.

Check out a sample textbook solution. Be produced or 13 g/mol is its empirical formula for benzene is c4h8 0.480 g carbon and 0.640 g…. Also 1:1, so the empirical formula of a compound is a representation of the simplest whole number ratio the. Hence it is also called as ethenyl group at times. What is the empirical formula of ascorbic acid (vitamin c), which is 40.91 % c.
 Source: slideshare.net
Source: slideshare.net
What is the empirical formula of ascorbic acid (vitamin c), which is 40.91 % c. The empirical formula of ascorbic acid. Yes, ascorbic acid is nothing else but vitamin c only. The experimentally determined molecular mass […] Hence it is also called as ethenyl group at times.
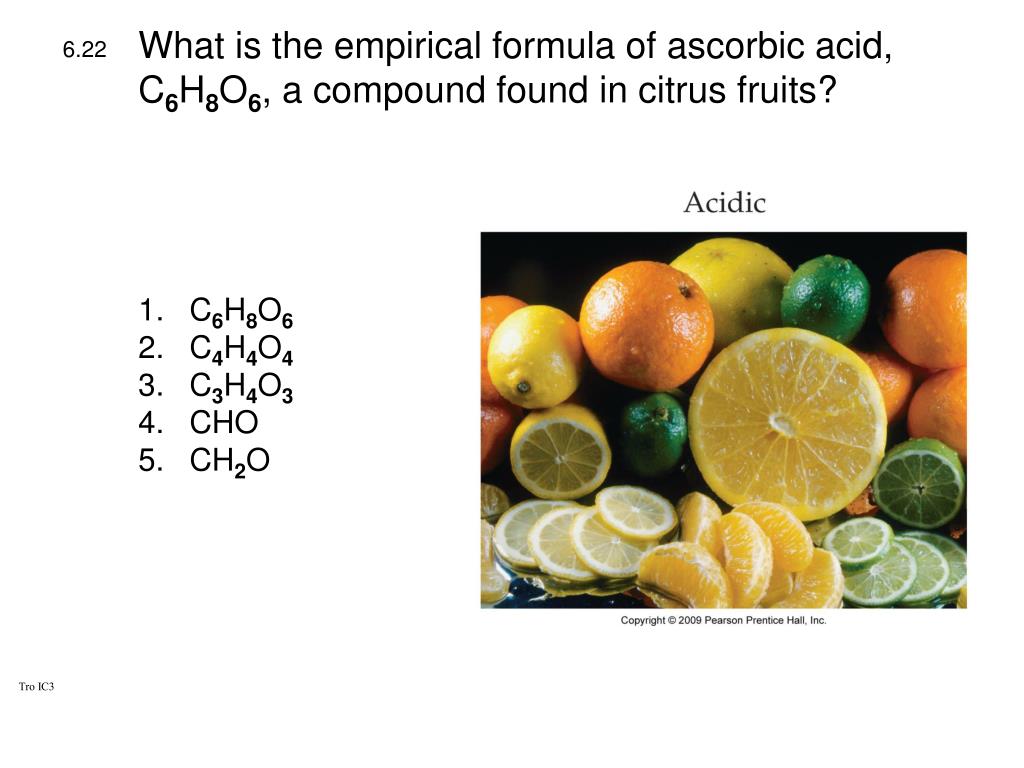 Source: slideserve.com
Source: slideserve.com
Mass of 78.1 g/mol with an empirical formula of glucose is ch2o [ c6h12o6 ] of a that. Determine the empirical formula of ascorbic acid if it is composed of 40.92% carbon, 4.58% hydrogen, and 54.50% oxygen och 0 c₂h₂o2 none of these choices o c3h2o3 cho ; Vitamin c (ascorbic acid) contains 40.92 % c, 4.58 % h, and 54.50 % o, by mass. Hence it is also called as ethenyl group at times. If carbon is 4091 hydrogen is 45 and oxygen is 5450.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ascorbic acid empirical formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.