Antacid neutralization science fair project
Antacid Neutralization Science Fair Project. This information is on the label of the antacid bottle. Your instructor will assign you an antacid to investigate. See more ideas about science projects, science fair, science. In this science project you used tums, but there are many other different brands of tablets sold to relieve heartburn.
 Antacid Science Fair Project Display Board · Super Science Fair Projects From super-science-fair-projects.com
Antacid Science Fair Project Display Board · Super Science Fair Projects From super-science-fair-projects.com
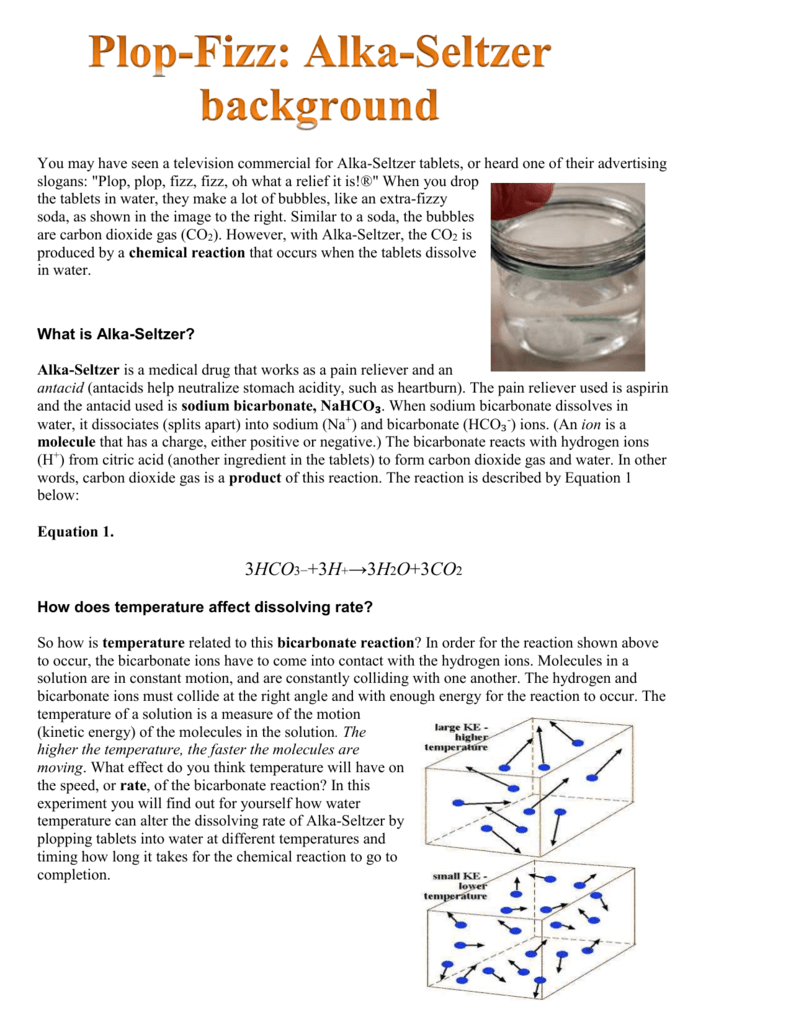
Antacid (weak base) + hcl (stomach acid) —> salts + h20 + c02 vinay kumar xii a. Record the concentration of the naoh solution being used. However, the inactive ingredients in the liquid and tablet form are. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), which is found in the stomach. Repeat this science project using a different brand or an antacid with a different active ingredient. Measure the ph using the ph probe start recording the ph.
Antacid science fair projects abstract reports on which antacid is best at neutralizing hydrocholoric aid.
Comparing the neutralization levels of different antacids j2117 objectives/goals by testing the effectiveness of different antacids in the same acid, i was able to. Your instructor will assign you an antacid to investigate. Science fair projects are a lot of work. This is how antacids function. Gold medal, 1st place award ($500 savings bond) from chicago drug and chemical association. Antacid neutralization science fair project.
 Source: room18sciencefair.weebly.com
Source: room18sciencefair.weebly.com
California state science fair 2017 project summary ap2/17 name(s) project number project title abstract summary statement help received harshini n. Touch device users can explore by touch or with swipe gestures. Antacid neutralization science fair project. This information is on the label of the antacid bottle. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), whic.
 Source: supplyme.com
Source: supplyme.com
When an acid and a base combine, the base neutralizes the acid in a chemical reaction. Additionally, while the antacid used in tums is calcium carbonate, there are many other antacids available. Gold medal, 1st place award ($500 savings bond) from chicago drug and chemical association. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), which is found in the stomach. The general neutralization reaction is:
 Source: markedbyteachers.com
Source: markedbyteachers.com
Additionally, while the antacid used in tums is calcium carbonate, there are many other antacids available. In this experiment, the neutralization capacity of commercial antacid tablet is done to determine the neutralization capacity of commercial antacid tablet by standardizing the hydrochloric acid, hcl against sodium hydroxide, naoh by using the standard naoh solution that we have prepared from experiment 1. This is a great demonstration to teach concepts of acids and bases, solubility, k sp and “antacid testing” consumer. This information is on the label of the antacid bottle. This is how antacids function.
 Source: pinterest.com
Source: pinterest.com
Put strips of paper into cabbage water. When an acid and a base combine, the base neutralizes the acid in a chemical reaction. However, the inactive ingredients in the liquid and tablet form are. Touch device users can explore by touch or with swipe gestures. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), whic.
 Source: pinterest.com
Source: pinterest.com
Add an indicator to the acid before adding any base or antacid. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), whic. Calcium salts may cause constipation. Add an indicator to the acid before adding any base or antacid. Science fair projects are a lot of work.
 Source: pinterest.com
Source: pinterest.com
A chemical reaction between the carbonate and hydrochloric acid may produce carbon dioxide gas. Spread strips out and allow to dry. A chemical reaction between the carbonate and hydrochloric acid may produce carbon dioxide gas. Repeat this science project using a different brand or an antacid with a different active ingredient. To determine which antacid could neutralize the most stomach acid.
 Source: pinterest.com
Source: pinterest.com
Record the name and the names and amounts of the active ingredients each tablet. Calcium carbonate 750 mg/tablet, aluminum hydroxide 160 mg/tablet+magnesium carbonate 105. Antacid (weak base) + hcl (stomach acid) —> salts + h20 + c02 vinay kumar xii a. Measure and record the mass of a single tablet of your assigned antacid. Heat cabbage in water for 15 minutes.
 Source: lab.en.mbtnua.com
Source: lab.en.mbtnua.com
Pink it is an acid. Your instructor will assign you an antacid to investigate. Victor aranki ,ensu kim 9th experiment 2. Antacid neutralization science fair project. Spread strips out and allow to dry.
 Source: pinterest.com
Source: pinterest.com
Antacid science fair projects abstract reports on which antacid is best at neutralizing hydrocholoric aid. Additionally, while the antacid used in tums is calcium carbonate, there are many other antacids available. In this science project you used tums, but there are many other different brands of tablets sold to relieve heartburn. In this experiment, the neutralization capacity of commercial antacid tablet is done to determine the neutralization capacity of commercial antacid tablet by standardizing the hydrochloric acid, hcl against sodium hydroxide, naoh by using the standard naoh solution that we have prepared from experiment 1. Other adverse effects from antacids include:
 Source: pinterest.com
Source: pinterest.com
Add an indicator to the acid before adding any base or antacid. Most people don’t realize that not all emails get delivered. Neutralization reaction of an antacid consumer chemistry introduction mix milk of magnesia (mom) with universal indicator and observe the dramatic rainbow of colors as the antacid dissolves in the simulated stomach acid! Gold medal, 1st place award ($500 savings bond) from chicago drug and chemical association. Record the name and the names and amounts of the active ingredients each tablet.

California state science fair 2017 project summary ap2/17 name(s) project number project title abstract summary statement help received harshini n. Comparing the neutralization levels of different antacids j2117 objectives/goals by testing the effectiveness of different antacids in the same acid, i was able to. Chemistry investigatory project comparitive study of commercial antacids a project report submitted by. Stomach acid is very dangerous. In this experiment, the neutralization capacity of commercial antacid tablet is done to determine the neutralization capacity of commercial antacid tablet by standardizing the hydrochloric acid, hcl against sodium hydroxide, naoh by using the standard naoh solution that we have prepared from experiment 1.
 Source: pinterest.com
Source: pinterest.com
Cut paper towel into strips. Here is a q&a page about creating a. This is a great demonstration to teach concepts of acids and bases, solubility, k sp and “antacid testing” consumer. Your package will be mailed to the manufacturer. Spread strips out and allow to dry.
 Source: super-science-fair-projects.com
Source: super-science-fair-projects.com
However, the inactive ingredients in the liquid and tablet form are. Cut paper towel into strips. Spread strips out and allow to dry. Antacid neutralization science fair project. Other adverse effects from antacids include:
 Source: studylib.net
Source: studylib.net
Comparing the neutralization levels of different antacids j2117 objectives/goals by testing the effectiveness of different antacids in the same acid, i was able to. Measure the ph using the ph probe start recording the ph. This is how antacids function. The step of the scientific process that i feel most confident in is. Victor aranki ,ensu kim 9th experiment 2.
 Source: slideshare.net
Source: slideshare.net
Measure the ph using the ph probe start recording the ph. Drain the leaves over a container. Here is a link to my science fair report. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), whic. Measure and record the mass of a single tablet of your assigned antacid.
 Source: pinterest.es
Source: pinterest.es
Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), whic. Antacids are weak bases (most commonly bicarbonates, hydroxides, and carbonates) that neutralize excess stomach acid and thus alleviate symptoms of heartburn. The general neutralization reaction is: Other adverse effects from antacids include: Calcium salts may cause constipation.
 Source: slideshare.net
Source: slideshare.net
Comparing the neutralization levels of different antacids j2117 objectives/goals by testing the effectiveness of different antacids in the same acid, i was able to. Purpose the purpose of the antacid neutralization science fair project is to determine which antacid works best at neutralizing hydrochloric acid (hci), whic. This information is on the label of the antacid bottle. If the liquid and tablet forms have the same amount of active ingredient (s), then, to first order, they should give similar results. Record the concentration of the naoh solution being used.
 Source: pinterest.com
Source: pinterest.com
Comparing the neutralization levels of different antacids j2117 objectives/goals by testing the effectiveness of different antacids in the same acid, i was able to. This is a great demonstration to teach concepts of acids and bases, solubility, k sp and “antacid testing” consumer. When an acid and a base combine, the base neutralizes the acid in a chemical reaction. Drain the leaves over a container. Repeat this science project using a different brand or an antacid with a different active ingredient.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title antacid neutralization science fair project by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.